Peri-operative Cardiac Assessment & Rationale for Use of Beta-Blockers. H. K. Chopra, Ravinder S. Sambi, Krishna C. K. , S. K. Parasher, (Prof) Manmohan Singh, Rakesh Gupta, Vijay Garg Department of Cardiology, Moolchand Medcity, Metro Heart Hospital, JROP Health Care, New Delhi , . Department of Cardiology, Government Medical College, Patiala, Punjab. Department of Cardiology R. D. Gardi Medical College, Ujjain, Madhya Pradesh |
|
|
Introduction:
The peri-operative consultation may represent the first detailed cardiovascular evaluation for the patient in years1. The initial detailed history, physical examination and ECG should help identify potentially serious underlying cardiac disease. Presence of anemia is also an important feature to assess. Clinical markers, which put patients at an intermediate risk are mild angina pectoris, recent myocardial infarction (MI), compensated heart failure (HF), diabetes mellitus (DM) and renal insufficiency (serum creatinine greater than or equal to = 2 mg/dL). Minor predictors of risk are advanced age, abnormal ECG, rhythm other than sinus, low functional residual capacity, prior history of stroke and uncontrolled hypertension.
The use of beta-blockers (BB) to reduce peri-operative cardiac events and mortality represents a major advance in peri-operative medicine for some patients at intermediate and high risk for cardiac events during non-cardiac surgery. Most studies report the effect of beta-blockade on peri-operative ischemia and have found a statistically significant reduction in ischemia among treated patients. Thus, physicians should try to begin BB therapy early enough so that it can be titrated appropriately. Thus administration of a therapeutic dose of BB prior to anesthesia and beta-blockade throughout the operation and postoperatively produced a significant reduction in ischemia, with an even greater benefit among high-risk patients2. The time required to accomplish this goal varies on the agent the root of administration and patient factors. Cardiac events are a frequent outcome in post-operative vascular surgery patients. Through the last decade, advances in pre-operative, intra-operative and post-operative management have resulted in better patient outcomes in non-cardiac surgery.
Epidemiology The prevalence of cardiovascular diseases increases with age; the number of people older than 65 in the United States are likely to increase 25% to 35% over the next 30 years3. The largest numbers of surgeries are also performed in the same age group and it has been estimated that number of non-cardiac surgical procedures will increase from the current 6 million to nearly 12 million per year in older people2. Each year 10% of the adult population undergoes non-cardiac surgery. One-third of these surgeries are performed in patients over 65 years and older. The overall mortality rate of all surgeries is 0.3%. For major surgeries the mortality rate is < 1% in patients less than 65 years, but increase to 5% for patients between 65-80 years. Also, patients over the age of 65 years are more likely to present for emergency surgeries than youngers patients (37% vs 17%)4. The peri-operative phase is a high- risk period for these patients to develop cardiac complications. Approximately 25% of these non-cardiac surgeries- major intra-abdominal, thoracic, vascular and orthopedic procedures; are associated with significant peri-operative cardiovascular events or deaths2. Cardiac events are the most common medical complication of surgery, occurring in 2% to 5% of patients undergoing non-cardiac surgery5 and as many as 30% of patients undergoing vascular surgery6,7. Myocardial ischemia is strongly linked with post-operative myocardial events and post-operative ischemia may increase the chances of post-operative myocardial events by 21 times2. Peri-operative cardiac events are associated with a mortality rate of 60% per event, prolonged hospitalization and higher costs6,8. This high cardiovascular morbidity and mortality in patients undergoing non-cardiac surgery can be reduced through a carefully tailored preoperative risk assessment and by initiating appropriate therapy. ACC/AHA practice guidelines on preoperative cardiovascular evaluation stress upon2:
Correspondence: Dr. H.K. Chopra, Senior Consultant Cardiologist, Moolchand Medicity, New Delhi,
Email: drhkchopra@yahoo.com
|
The preoperative cardiac evaluation is patient specific and tailored to the patient’s specific needs and problems. Success of peri-operative assessment is reliant upon strong communication between patients and health care professionals. BB usage prior to surgery is dependent upon urgency of non-cardiac surgery, the patient’s risk factors and specific surgical considerations. Coronary revascularization before the non-cardiac surgery may be helpful for any high risk patients, but that is an exception rather than the rule1. Table 1: Stepwise Approach for Peri-operative Cardiovascular Evaluation for Non-cardiac Surgery.
|
Assessing the patient’s medical status
The peri-operative consultation may represent the patient’s first cardiovascular evaluation. The initial history, physical examination and ECG should help identify potentially serious underlying cardiac disease if any1. Other important features to consider are the patient’s age, sex, functional residual capacity, hypertension, DM, coronary artery disease (CAD), chronic obstructive pulmonary disease (COPD) and other factors. For example, a risk asymptomatic patient who runs for 30 minutes daily may not require as much evaluation while, as a sedentary patient with unclear clinical features suggestive of increased peri-operative risk1. Other factors that help determine cardiac risk include co-morbid illnesses, such as: DM, peripheral vascular disease, renal dysfunction and COPD and the type of surgery.
On the basis of multivariate analysis, a number of risk indices have been developed by the American Society of Anesthesiologists (ASA) Score10, Goldman’s risk index (1997)11, Detsky’s Modified risk index (1997)12, the Preoperative Canadian Cardiovascular Society Index for Angina Level13, The Revised Cardiac Index, APACHE scores and Pre-operative Guidelines by the AHA, help physicians stratify the risk for cardiac complications. Although these cardiac indices provide useful clinical information about risk, their overall accuracy is still considered limited14.
ACC/AHA using collected observational data and expert opinion developed a stepwise approach to cardiac assessment of patients-see figure 1. This stepwise strategy relies on the assessment of clinical markers, prior coronary evaluation and treatment, functional residual capacity, and surgery-specific risk, which are well known clinical risk predictors. The clinician must consider 2 factors when assessing the patient's cardiovascular risk: (1) The type of surgery and (2) The hemodynamic stress associated with the procedure1.
Generally, the more extensive the surgical procedure, the greater is the physiological stress with more significant the post-operative pain and the greater the incidence of cardiac complications. Cardiac risks of surgery include non-fatal MI and fatal cardiac events. The surgeries are stratified on the basis of cardiac risk involved- high, intermediate or low risk (Table 2)1.
| Table 2: Cardiac Risk Stratification for Non-cardiac Surgical Procedures. | ||||||||||||||||
|
|
S. No. |
Predictor |
Examples |
1 |
Major |
|
2 |
Intermediate |
|
3 |
Minor |
|
MI: myocardial infarction, LVH: left ventricular hypertrophy, LBBB: left bundle-branch block, RBBB: right bundle-branch block, AF: atrial fibrillation.
Using the ACC/AHA algorithm for stepwise approach to preoperative cardiac assessment (Refer to Figure 1)1,2.
Step 1: Emergency non-cardiac surgery.
Emergencies may not allow preoperative evaluation, and in such cases, postoperative risk stratification may be appropriate.
Step 2: Coronary revascularization in the last 5 years.
If yes and clinically the patient is stable without any recurrent symptoms/signs of ischemia, one can proceed with surgery without any further evaluation.
Step 3: Coronary evaluation in the past 2 years.
If so and findings were favorable, no need to repeat testing, unless patient has new or fresh symptoms.
Step 4: Patient has unstable coronary syndrome or a major clinical predictor of risk.
In elective non-cardiac surgery, delay or cancel the surgery until the problem has been identified and treated.
Step 5: Patient has intermediate clinical predictors of risk.
Consider functional residual capacity and level of surgery-specific risk to identify patients who will benefit from further noninvasive testing. In this group, patients who are eventually cleared for surgery should undergo post-operative risk stratification and risk reduction.
Step 6: Patient with intermediate clinical predictors of risk with moderate or excellent (> METs) functional capacity.
Such a patient is generally fit for intermediate-risk surgery. Otherwise further noninvasive testing is often considered.
|
Non-cardiac surgery is generally safe. Further evaluation may be appropriate for patients without clinical markers with poor functional capacity, who are facing higher risk surgery.
Step 8: Further noninvasive testing puts patient into high risk.
Consider invasive testing such as coronary angiography. Subsequent treatment plan may include cancellation or delay of surgery; coronary revascularization followed by non-cardiac surgery or intensified care.
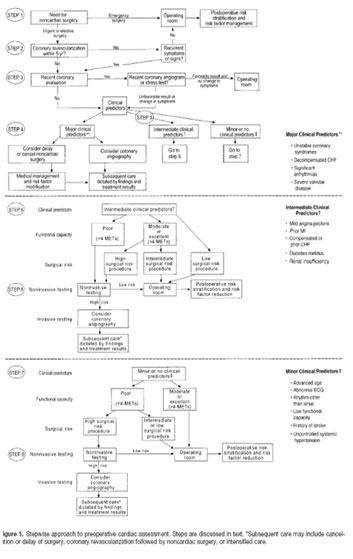 |
Figure 1: Stepwise approach to pre-operative cardiac assessment. Steps are discussed in the text. |
Recommendations For Supplemental Evaluation
Patients who fall into high risk status as seen in Table 5 need more immediate workups and will need to be targeted to specific conditions. In some cases revascularization by PCI may be warranted before non-cardiac surgery. There are many excellent non-invasive tests for cardiac disease. One of the most helpful has been the use of echocardiogram. This test allows evaluation of wall motion abnormalities, ejection fractions and possible intra-cardiac shunts. It is important to remember that most non-invasive cardiovascular testing results are very technically dependent. The use of Echocardiograms in the evaluation of patients with chronic stable angina has class I indications1,9.
Table 5: Non-invasive testing in preoperative patients (if any 2 factors are present).
S. No. |
Factors |
1 |
Intermediate clinical predictors are present (Canadian Class I or 2 angina, prior MI based on history of pathologic Q waves, compensated or prior HF or DM). |
2 |
Poor functional capacity (< 4 METs). |
3 |
High surgical risk procedure. |
HF: heart failure, METs: metabolic equivalents, MI: myocardial infarction, DM: diabetes mellitus.
Recommendations for tests
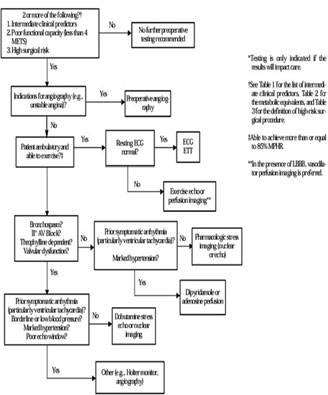
Recommendations for the use of these tests in a preoperative setting with summaries of evidence are given in the ACC/AHA practice guidelines. In most ambulatory patients, exercise ECG is the test of choice which provides both an estimate of functional capacity and detects myocardial ischemia through changes in the ECG and hemodynamic response. Exercise echocardiography or exercise myocardial perfusion imaging should be considered for patients who have important abnormal ECG’s such as: left bundle branch block (LBBB), left ventricular hypertrophy with ‘strain’ pattern, or digitalis effect. The sensitivity and specificity of exercise thallium scans in the presence of LBBB are reported to be 78% and 33% respectively and overall diagnostic accuracy varies from 36% to 60%2.
Dipyridamole myocardial perfusion imaging testing and dobutamine echocardiography are two of the most common non-exercise stress tests that are used for patients unable to undergo exercise stress testing. Dobutamine should not be used in patients with serious arrhythmias or severe hypertension or hypotension. IV dipyridamole should be avoided in patients with significant bronchospasm or critical carotid artery disease. For a better evaluation of valvular disease, the echocardiographic stress test is a favored test. Mantha S et al, in a meta-analysis, found that all 4 tests — dobutamine stress echocardiography, ambulatory electrocardiography, radionuclide ventriculography, and dipyridamole thallium scanning — had a similar predictive value21. An algorithm (Figure 2) by ACC/AHA Task Force helps the clinicians select the most appropriate test in various clinical situations.
|
Figure 2: Supplemental pre-operative evaluation. |
 |
| Recommending invasive evaluation: Coronary angiography
In patients with major clinical predictors or evidence of residual ischemia after recent MI, coronary angiography is often indicated. Additionally, patient subsets with intermediate or minor clinical risk predictors, who are found at high risk through noninvasive testing, also are considered for invasive evaluation22. A stepwise approach to preoperative assessment allows judicious use of both noninvasive and invasive tests while preserving a low rate of cardiac complications23. DISEASE-SPECIFIC MANAGEMENTHypertension Hypertension affects 60-80 million people in the United States and treatment reduces death rates from strokes and CHD. Approximately 40% of patients who are aware they have hypertension are either not treated or inadequately treated with pharmacological therapy1. Only a small number of patients are treated properly. Peri-operative assessment is a unique opportunity to identify these patients and initiate proper therapy. Hypertension is a major risk factor of cardiovascular disease24. Additionally, several studies have documented exaggerated intra-operative blood pressure fluctuations accompanied with ECG evidence of myocardial ischemia25-28. This effect could be modified by treatment26-31. In the peri-operative period, poorly controlled hypertension is associated with an increased incidence of ischemia, MI, left ventricular dysfunction, arrhythmia and stroke1,2. There is sufficient body of evidence for value of effective preoperative control of hypertension27,28,31 and antihypertensive medications should continue during the peri-operative period. Stage 2 hypertension (systolic blood pressure of ≥160 mm Hg and diastolic blood pressure of ≥100 mm Hg) should be well controlled before elective surgery1. An effective control should be achieved over several days to weeks of pre-operative outpatient treatment. In more urgent surgeries, rapid-acting agents should be used for achieving control within minutes or hours. BBs appear to be particularly attractive agents. Several reports have shown that use of pre-operative beta-blockade leads to effective control of exaggerated blood pressure fluctuations and reduction in the number and duration of peri-operative ischemic events26-31. Pre-operative beta-blockade also results in a reduction of post-operative atrial fibrillation32, mortality and incidence of cardiac events in patients undergoing non-cardiac surgery who have or are at risk for CAD33, 34. |
Heart failure
Heart failure in patients undergoing non-cardiac surgery is associated with a poor outcome. Goldman et al11 found that presence of third heart sound or signs of HF were associated with an increased risk during non-cardiac surgery. Pulmonary edema was identified as an important risk factor by Detsky et al35. Identifying the etiology of HF is very important, since it affects the patient’s treatment and level of risk2. Therapy is aimed at reducing ventricular filling pressures in addition to improving cardiac output. Medications proven to show both a morbidity and mortality benefit include ACE inhibitors, BBs, and spironolactone. Digoxin and diuretics have been shown to improve morbidity rates without reducing mortality rates1,2.
History of pre-operative congestive HF increases the risk of pulmonary edema from 3% in NYHA class I to 25% in patients with class IV. Though in 50% of patients who develop post-operatively congestive HF, there is no prior history of HF and most of them develop congestive HF in the first hour of completion of surgery. These patients have to be managed aggressively post-operatively with diuretics and medical management36.
Valvular heart disease
Identification of patients with Valvular heart disease is extremely important because of volume shifts and other hemodynamic changes during surgery. Aortic stenosis and ischemic mitral regurgitation are more common forms of valvular heart disease seen in preoperative patients. In most instances the use of ETT or echocardiograms can help direct the physician to the best treatment options for these patients. Patients with valvular heart disease especially severe aortic stenosis (AS) have increased risk for surgery as they have fixed cardiac output and do not tolerate general or spinal anesthesia due to vasodilatation. Patients with moderate to severe mitral stenosis also tolerate surgery poorly. Patients with aortic or mitral regurgitation with preserved left ventricular ejection fraction tolerate surgery fairly well. But of course, patients with valvular heart disease should receive infective endocarditis prophylaxis36-39.
Cardiomyopathy
As patients reach older ages peri-operative heart failure is becoming more common. Careful evaluation in these patients is necessary because of critical changes in volume states and HF becomes an issue. An increased incidence of peri-operative HF has been seen with dilated and hypertrophic cardiomyopathy11,40,41. In such instances, improving the hemodynamic status and optimizing medical therapy and surveillance in the post-operative period are the focus of management. An estimate of hemodynamic reserves helps clinicians to anticipate any complications during and after the surgery.
Rhythm and conduction abnormalities
Any electrical abnormality in the heart needs to be evaluated for underlying disease, drug toxicity and metabolic pathology. Symptomatic or hemodynamically unstable arrhythmias should be treated; indications for anti-arrhythmic therapy and cardiac pacing are identical to those in the non-operative setting. Frequent ventricular premature contractions and/or asymptomatic non-sustained ventricular tachycardia have not been documented to raise the risk of nonfatal MI or cardiac death in the peri-operative period42,43.
Patients with arrhythmias may tolerate surgery poorly as supra-ventricular arrhythmias need to be rate controlled while ventricular arrhythmias need to be evaluated by a cardiologist prior to surgery. Those with first degree and Mobitz type 1 atrioventricular block tolerate surgery well but Mobitz type 2 and third degree atrioventricular block require intracardiac pacing42,43.
Implantable pacemakers or ICDs
Each year, more than 200,000 patients receive a permanent pacemaker and more than
60,000 receive an implantable defibrillator. The presence of these devices influences the peri-operative management. Evaluation of a pacemaker or an ICD depends on the urgency of the surgery — whether a pacemaker has unipolar or bipolar leads, whether electrocautery is bipolar or unipolar, the distance between cautery and pacemaker and whether the patient is pacemaker dependent42,43. Detailed recommendations are given in the ACC/AHA practice guidelines1,2.
|
Peri-operative therapy or previous coronary revascularization
Coronary artery bypass grafting is rarely indicated to enable patients endure the stress of non-cardiac surgery. Analysis of CASS44 database showed that, the cardiac risk associated with non-cardiac procedures involving the thorax, abdomen, arterial vasculature, and head and neck was significantly lower in patients who had undergone prior CABG. According to ACC/AHA guidelines and indications for CABG45, patients undergoing elective non-cardiac surgery of high- or intermediate- risk (Table 2) found to have high-risk CAD and in whom long-term outcome would likely be improved by CABG, should undergo prior revascularization.
Percutaneous coronary intervention
There are no well-designed comparative studies on outcomes after non-cardiac surgery, between pre-operative percutaneous coronary intervention (PCI) and medical therapy. Few observation studies have suggested some benefit of pre-operative PCI46-50. In the absence of any concrete evidence, indications for PCI in the peri-operative setting are similar to the ACC/AHA guidelines for use of PCI51.
In the light of uncertainty on how much time should be kept between PCI and the non-cardiac procedure, delaying the surgery for at least 1 week after balloon angioplasty is considered a sound strategy. In case, a coronary stent is placed, one should wait ideally for 4 to 6 weeks before the non-cardiac surgery. This period allows completion of re-endothelialization of the stent and manifestation of effects of dual antiplatelet therapy52.
Peri-operative medical therapy
Patients undergoing non-cardiac surgery should be receiving optimal medial therapy, both peri-operatively and long-term according to their underlying cardiac condition22. Until recently, methods to reduce the incidence of these complications depended upon preoperative evaluation of risk, followed by additional noninvasive or invasive tests or revascularization procedures, as appropriate12.
Several randomized trials in the recent past have examined the impact of beginning medical therapy just before surgery on reducing cardiac events. These studies have evaluated BBs, alpha agonists, nitroglycerin, and calcium channel antagonists. Since a number of clinical diseases increase the risk of peri-operative cardiac complications, optimal control of these factors is also an important (Table 2) area of focus. Wherever appropriate use of aspirin, clopidogrel, ACE inhibitors and statins are encouraged to improve the underlying cardiac problems22. A strong body of evidence suggests a direct link between myocardial ischemia and postoperative myocardial events53,54. A study by Landesberg G, et al, found a 21-fold increase in the odds of post-myocardial events due to postoperative ischemia55. Peri-operative and post-operative ischemia or MI are most likely caused by an increase in myocardial oxygen demand in patients with CAD and those in whom coronary plaque ruptures may be triggered56. Increased catecholamine levels and prothrombotic tendencies play an important role in these events.
CLINICAL EVIDENCE FOR EFFICACY OF BETA-BLOCKERS
Most studies reporting the effect of beta-blockade on peri-operative ischemia have found a statistically significant reduction in ischemia among treated patients. Wallace et al60 (Table 6) in a subset analysis of data from the study by Mangano et al33 reported less frequent peri-operative myocardial ischemia in atenolol-treated patients. In this study BBs were given pre-operatively and up to 7 days after the surgery. Main findings of this study were: 1) All cause mortality at 2 years was 9% compared to 21% (p=0.02) in the control group; 2) Cardiac death at 2 years was 4% compared to 12% (p=0.03); 3) Post-operative ischemia was 24% compared to 39% (p=0.03) in the control group.
Stone et al26 suggested a similar effect of beta-blockade on Holter monitor-documented myocardial ischemia. In this study, patients were given a single small oral dose of a BB, 2 hours before induction of anesthesia. The incidence of postoperative MI was 2% compared to 28% of the control group (p < 0.001). However, this study was open to debate since the authors did not report the types of surgery nor did it compare to the baseline patient characteristics. Raby et al58 also found a significant beneficial effect of beta-blockade using a continuous infusion of esmolol in high-risk patients undergoing vascular surgery. The documented incidence of post-operative myocardial ischemia, which was 33% in the treated group, compared to 73% (p < 0.05) in the control group.
Two studies have reported significant improvement in patient’s outcome on cardiac events and cardiac morality due to BBs. In the first study of male veterans undergoing major non-cardiac surgery, Mangano et al33, reported a relative reduction in all-cause mortality of nearly 55% at 2 years (67% reduction at year 1, 48% at year 2). However, patients who were in the BB group had less CAD at study entry, were on ACE-inhibitors more frequently and were less likely to have BBs discontinued peri-operatively, possibly leaning the results in favor of treatment group61,62.
Poldermans et al34 found an even greater benefit of beta-blockade among the high-risk patients. This team analyzed data of enrolled patients who were to undergo vascular surgery and had
|
myocardial ischemia documented by dobutamine echocardiography with an estimated rate of major peri-operative cardiac events of 28%. The entire patient cohort had experienced a 90% reduction in cardiac death or nonfatal MI by 30 days. The rates of cardiac death (3.4 vs. 17%; p = 0.02) and non-fatal MI (0%vs. 17%; p < 0.001) were lower in the beta-blocker treated group. Bisoprolol orally in this study was started an average of 37 days before surgery and continued 30 days after it. Intravenous metoprolol was used to target heart rate if patients were not taking BBs peri-operatively.
Follow-up care did not include additional therapy (i.e. cardiac catheterization, revascularization), raising concerns that the research algorithm failed to reflect optimal clinical practice63,64. However, if the true rate of events in treated patients is low, the risk associated with revascularization may outweigh any benefit65. The greater benefit seen in Poldermans study34 may also be due to the fact that the study did not enroll patients who were receiving BBs. BBs naïve patients may have a different response to peri-operative beta-blockade (Table 6)9.
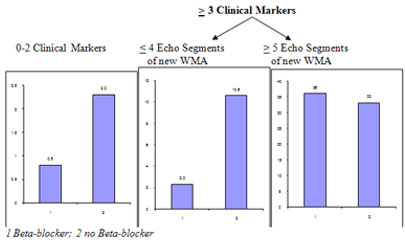
Boersma et al analyzed a cohort of 1,351 consecutive patients enrolled in a randomized trial of bisoprolol59. Eighty three percent of the 45 patients, who had peri-operative cardiac death or nonfatal MI, had fewer than 3 clinical risk factors. Among this subgroup, patients receiving BBs had a lower risk of cardiac complications (0.8%) than those not receiving BBs (2.3%). In patients with 3 or more risk factors (15%), those taking BBs who had a DSE demonstrating 4 or fewer segments of new wall motion abnormalities, had a significantly lower incidence of cardiac complications (2.3%) compared with those not receiving BB therapy (10.6%). Moreover among patients with more extensive ischemia, there was no difference in the incidence of cardiac events. Therefore, BB therapy was beneficial in all but the subsets of patients with more extensive ischemia (Figure 3).
Pasternack et al66 used oral metoprolol immediately before surgery and followed it up using the intravenous formulation during abdominal aortic aneurysm. Compared to 18% in the controlled group, only 3% suffered incidence of an acute MI. In a later report the same authors reported less intraoperative ischemia in patients treated with oral metoprolol before peripheral vascular surgery29.
|
PATIENT SELECTION FOR BETA-BLOCKER THERAPY
Comparative studies of peri-operative beta-blocker therapy have been performed largely in selected patient population with peri-operative cardiac risk higher than that of the general population of surgical patients (Table 7)9. Thus, clinicians must seek data from studies that included patients
| Table 6: Peri-operative Beta-Blocker Therapy. | |||||||||||||||
|
|||||||||||||||
Which beta-blocker should be selected and why? |
|
Table 7: Randomized controlled trials of the effectiveness of peri-operative beta-blockade
Source Beta-blocker regimen Findings (postoperative ischemia/other)
Mangano et al33 1996; Atenolol, 5-10 mg intravenously No differences in in-hospital cardiac or mortality
Wallace et al60 1998 30 min before and after surgery outcomes. All-cause mortality at 2 years: 9% vs 21%
and 50-100 mg/d by mouth (P=.02); cardiac death at 2 years: 4% vs 12% (P=0.3);
throughout the hospital stay postoperative ischemia: 24% vs 39% (P=.03)
(up to 7 days)
Stone et al26 1988 Labetalol, atenolol, oxprenolol; patients Postoperative MI: 2/89 (2%) vs 11/39 (28%) untreated
randomized to control, labetalol (P<.001)
(100mg by mouth), atenolol (50 mg by
mouth), or oxprenolol (20 mg by mouth)
given before induction of anesthesia
Raby et al58 1999 Esmolol, intravenous for 48 hours Postoperative myocardial ischemia: 33% vs 73%
postoperatively (P < .05)
Poldermans et al34 1999 Bisoprolol, 5-10mg/d by mouth begun Reduced incidence of perioperative cardiac death and
an average of 37 days preoperatively and nonfatal MI. Cardiac death: 3.4% vs 17% (P = .02);
continued for 30 days postoperatively nonfatal MI: 0% vs 17% (P < .001)
or infrainguinal arterial reconstruction
Pastenack66 1987 50mg PO metoprolol preoperatively Acute MI: 3.1% vs. 17.6%
Urban85 2000 Esmolol intravenous within 1hr after Post operative ischemia: 6% vs 15% (p=NS) surgery, titrate to heart rate <80 bpm Post operative MI: 2% vs 6%(p=NS) Change to metoprolol morning of the 1st post operative day. Titrate to heart <80 bpm for the next 48hrs, then continue dose till discharge.
Table 8: Risk factors of Peri-operative Cardiac complications and indications for beta-blocker therapy.
Risk Variables |
Beta-blocker indicated |
Clinical featuresIschemic heart disease Heart failure Diabetes Renal dysfunction Functional statusPoor functional status (inability to walk 4 blocks or climb 2 flights of stairs) |
Yes Yes Yes Uncertain, but probable if renal insufficiency is due to diabetes or vascular disease |
Surgery |
No Clinical Risk Factors |
1 or more Clinical Risk Factors |
CAD or High Cardiac Risk |
Patients Currently on Beta Blockers |
Vascular |
Class IIb, Level of Evidence: B |
Class IIa, Level of Evidence: B |
Patients found to have myocardial ischemia on pre-operative testing: Class I, Level of Evidence: B* |
Class I, Level of Evidence: B |
|
|
|
Patients without ischemia or no previous test: Class IIa, Level of Evidence: B |
|
Intermediate risk |
|
Class IIb, Level of Evidence: C |
Class IIa, Level of Evidence: B |
Class I, Level of Evidence: C |
Low risk |
|
|
|
Class I, Level of Evidence: C |
Initiation and titration of beta-blockers
Although questions remain regarding the optimal dosing for BB therapy in the peri-operative phase studies conducted so far have targeted to achieve sympatholytic effect before induction of anesthesia. Thus, physicians should try to begin therapy early enough so that it can be titrated appropriately. The time required to accomplish this goal varies on the agent the root of administration and patient factors. The most frequently used parameter to achieve adequate beta-blockade has been heart control. However, it is not yet clear whether it is only heart rate control or also its positive effects on myocardial oxygen supply and demand that cause reduced mortality or are just markers. Perhaps the true beneficial effects result from reduced peri-operative arrhythmias, reduced likelihood of plaque rupture, or reduced risk of thrombus formation the anti-coagulant effects of BBs. Although most of the studies achieved a heart rate less than 80/minute, others have recommended titrating beta-blockade till a heart rate of 65/minute or less is achieved.
Several recent randomized, prospective studies suggest the use of BB peri-operatively reduces the incidence of cardiac morbidity and mortality in high-risk patients. In accordance with these findings, the AHA and the ACC (Table 9)9 have published guidelines recommending peri-operative BB administration to achieve a heart rate of 50-60 beats per minute. And it has also been determined that tachycardia is associated with higher mortality in post-operative ICU patients.
Selecting patients for additional cardiac risk stratification
Studies on effectiveness of beta-blockade, especially the results of the study by Poldermans et al34, have made some authors wonder whether risk stratification is really necessary63. However, BBs alone may not reduce the risk of post-operative cardiac events below thresholds as suggested in the ACC/AHA practice guidelines2. Boersma et al59 in their study found that patients who were in the highest risk strata and received BBs continued to have an estimated cardiac event rate of 14%; these authors suggested that patients with more than 3 clinical predictors be referred for additional risk stratification using non-invasive testing. Thus, it can be inferred that although BBs may raise the threshold at which clinicians refer patients for additional testing, the era of risk stratification is not over.
Adverse effects of peri-operative beta-blockade
Stone et al26 reported high rate of bradycardia in BB treated patients, 50% of whom required atropine therapy. However, the vague descriptions and more general problems with the study’s design made it difficult to interpret the significance of these events in the clinical practice. Adverse events related to the use of BBs in other reviewed studies were infrequent and did not require discontinuation of the medication33. Similar rates of adverse effects have been noted in studies examining beta-blockade in patients undergoing cardiac surgery. Bayliff et al69 reported more frequent post-operative bradycardia, hypotension, and bronchospasm with the use of propranolol for the prevention of post-operative arrhythmias. The use of peri-
|
operative beta-blockade in patients who had not been receiving long-term beta-blocker may also pose an additional risk, in that withdrawal of beta-blockers may lead to adrenergic hypersensitivity and possibly worsen outcomes. A recent prospective observational study noted that patients who were not receiving beta-blockers on a long-term basis but who discontinued peri-operative use immediately after surgery had a markedly increased risk for postoperative MI70. This effect was not observed in randomized trials of beta-blockade that used shorter treatment regimens26,58 and need to be confirmed by larger studies.
PERI-OPERATIVE SURVEILLANCE
While much attention has been focused on the preoperative preparation of high-risk patient, intra-operative and post-operative surveillance for myocardial ischemia and infarction, arrhythmias and venous thrombosis should also lead to reductions in morbidity. Post-operative myocardial ischemia has been shown to be the strongest predictor of peri-operative cardiac morbidity and is rarely accompanied by pain. Hence, it is likely to go untreated till the development of overt symptoms of cardiac failure. A peri-operative MI is associated with 30% to 50% peri-operative mortality with reduced long term survival2. Therefore, it is important to identify patients who develop a peri-operative MI and to treat them aggressively to reduce both short- and long-term risks. The key areas of peri-operative surveillance outlined by ACC/AHA practice guidelines are 1) Intra-operative and postoperative use of pulmonary artery catheters; 2) Intra-operative and postoperative ST segment monitoring; 3) Surveillance for peri-operative MI; and 4) Arrhythmia/ Conduction disease disorders.
POSTOPERATIVE AND LONG-TERM MANAGEMENT
Cardiac events are a frequent outcome in post-operative vascular surgery patients. Through the last decade, advances in pre-operative, intra-operative and postoperative management have resulted in better patient outcomes in non-cardiac surgery. However, even after optimal peri-operative management some patients will have peri-operative MI that is associated with a high mortality rate71. Angioplasty should be considered for patients who experience a symptomatic, peri-operative, ST-segment elevation MI as a result of sudden thrombotic coronary occlusion. Pharmacological therapy with aspirin should be started as early as possible and a BB and ACE inhibitor may also be beneficial1. Patients who develop acute MI in the peri-operative phase should be offered careful medical evaluation for residual ischemia and left ventricular function.
The recommendation of secondary risk reduction is also appropriate in patients whom cardiovascular abnormalities are detected during preoperative evaluation. Patients with abnormal cholesterol levels benefit from pharmacological agents, such as statins. It is important to note that most of the patients who have known or newly detected CAD during their peri-operative evaluations will not have any events during elective non-cardiac surgery.
The cardiovascular assessment of patients undergoing non-cardiac surgery has been investigated intensively over the past 10 years and published guidelines. In all studies, it was found that BBs reduce mortality and morbidity significantly in ischemic heart disease even for patients who have relative contraindication. They further increase stability of coronary atherosclerosis plaques or increase the threshold for ventricular fibrillation in the presence of ischemia. Many trials have been conducted to improve mortality and morbidity pre-operatively using BB, calcium channel blockers, nitrates and ACE inhibitors. But the only one which shows encouraging results is the use of BB by reducing the heart rate, decreasing contractility and reducing the effect of surged catecholamine which reduces oxygen consumption.
Heart protection is essentially required during stressful condition. Beta-blockers if started pre-operatively and continued intra-operatively and post-operatively, play a major role in protecting the heart and thus reduce the morbidity and mortality by more than 50%.
1. Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for peri-operative cardiovascular evaluation for non-cardiac surgery- Executive Summary. A Report of the ACC/AHA Task Force on Guidelines on peri-operative cardiovascular evaluation for non-cardiac surgery. Circulation 2002; 105: 1257-67.
2. Eagle KA, Berger PB, Calkins H, et al. ACC/AHA guideline update for perioperative cardiovascular evaluation for noncardiac surgery. A report of the ACC/AHA. Task Force on Practice Guidelines (Committee to Update the 1996 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). 2002. www.acc.org/clinical guidelines/perio/dirIndex.htm
3. Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990; 72: 153-184.
4. Beliveau MM, Multach M. Peri-operative care of the elderly patients. Med Clin North Am 2003; 87: 273-89.
5. Lee TH, Marcantonio ER, Mangione CM, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circ J Am Heart Assoc. 1999; 100: 1043-1049.
6. Lee TH. Reducing cardiac risk in noncardiac surgery. N Engl J Med. 1999; 341: 1838-1840.
7. Poldermans D, Boersma E, Bax JJ, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med. 1999; 341: 1 789-1794.
8. Goldman L. Multifactorial index of cardiac risk in noncardiac surgery: ten-year status report. J Cardiothorac Anesth. 1987; 1: 237-244.
9. Lee AF, Joshua AB, Kenneth AB, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery. A Report of the ACC/AHA Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery). J Am Coll Cardiol, 2007; 50: 159-242.
10. American Society of Anesthesiologists. New classification of physician status. Anesthesiology. 1963; 24: 111.
11. Goldman L, Caldera DL, Nussabaum SR, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med. 1977; 297: 845-850.
12. American College of Physicians. Guidelines for assessing and managing the perioperative risk from coronary artery disease associated with major noncardiac surgery. Ann Intern Med. 1997; 127: 309-312.
13. Campeau L. Letter: Grading of angina pectoris. Circ J Am Heart Assoc. 1976; 54: 522-523.
14. Gilbert K, Larocque BJ, Patrick LT. Prospective evaluation of cardiac risk indices for patients undergoing noncardiac surgery. Ann Intern Med. 2002; 133 (5): 356-359.
15. Reilly DF, McNeely MJ, Doerner D, et al. Self-reported exercise tolerance and the risk of serious perioperative complications. Arch Intern Med. 1999; 159: 2185-2192.
16. Older P, Hall A, Hader R. Cardiopulmonary exercise testing as a screening test for perioperative management of major surgery in the elderly. Chest. 1999; 116: 355-362.
17. Bartels C, Bechtel JF, Hossmann V, et al. Cardiac risk stratification for high-risk vascular surgery. Circ J Am Heart Assoc. 1997; 95: 2473–2475.
18. Hlatky MA, Boineau RE, Higginbotham MB, et al. A brief self-administered questionnaire to determine functional capacity (the Duke Activity Status Index). Am J Cardiol. 1989; 64: 651-654.
19. Tarhan S, Moffitt EA, Taylor Wf, et al. Myocardial infarction after general anaesthesia. Anesth Analg, 1977; 56: 455-461.
20. Lindenauer PK, Pekow O, Wang K, et al. Peri-operative beta-blocker therapy and mortality after major non-cardiac surgery. N Engl J Med 2005; 353: 349-361.
21. Mantha S, Roizen MF, Barnard J, et al. Relative effectiveness of four preoperative tests for predicting adverse cardiac outcomes after vascular surgery: a meta-analysis. Anesth Analg. 1994; 91: 46-53.
22. Mukherjee D, Eagle KA. Perioperative cardiac assessment of noncardiac surgery. Eight surgery. Eight steps to the best possible outcome. Circ J Am Heart Assoc. 2003; 107: 2771-2774.
|
24. The fifth report of the Joint National Committee on Detection, Evaluation, and Treatment of High Blood Pressure (JNC V). Arch Intern Med. 1993; 153: 154-183.
25. Charlson ME, MacKenzie CR, Gold JP, et al. Preoperative characteristics predicting intraoperative hypotension and hypertension among hypertensives and diabetics undergoing noncardiac surgery. Ann Surg. 1990; 212: 66-81.
26. Stone JG, Foex P, Sear JW, et al. Myocardial ischemia in untreated hypertensive patients: effect of a single small oral dose of a beta-adrenergic blocking agent. Anesthesiology. 1988; 68: 495-500.
27. Stone JG, Foex P, Sear JW, et al. Risk of myocardial ischaemia during anaesthesia in treated and untreated hypertensive patients. Br J Anaesth. 1988; 61: 675-679.
28. Prys-Roberts C, Meloche R, Foex P. Studies of anaesthesia in relation to hypertension, I: cardiovascular responses of treated and untreated patients. Br J Anaesth. 1971; 43: 122-137.
29. Pasternack PF, Grossi EA, Baumann FG, et al. Beta-blockade to decrease silent myocardial ischemia during peripheral vascular surgery. Am J Surg. 1989; 158: 113-116.
30. Cucchiara RF, Benefiel DJ, Matteo RS, et al. Evaluation of esmolol in controlling increases in heart rate and blood pressure during endotracheal intubation in patients undergoing carotid endarterectomy. Anesthesiology. 1986; 65: 528-531.
31. Magnusson J, Thulin T, Werner O, et al. Haemodynamic effects of pretreatment with metoprolol in hypertensive patients undergoing surgery. Br J Anaesth. 1986; 58: 251-60.
32. Jakobsen CJ, Bille S, Ahlburg P, et al. Perioperative metoprolol reduces the frequency of atrial fibrillation after thoracotomy for lung resection. J Cardiothorac Vasc Anesth. 1997; 11: 746-751.
33. Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group [published erratum appears in N Engl J Med. 1997; 336: 1039]. N Engl J Med. 1996; 335: 1713-1720.
34. Poldermans D, Boersma E, Bax JJ, et al, for the Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. N Engl J Med. 1999; 341: 1789-1794.
35. Detsky AS, Abrams HB, McLaughlin JR, et al. Predicting cardiac complications in patients undergoing non-cardiac surgery. J Gen Intern Med. 1986;1: 211-219.
36. Goldman L, Caldera DL, Southwick FS, et al. Cardiac Risk factors and complications in non-cardiac surgery. Medicine 1978; 57: 357-370.
37. Reyes VP, Raju BS, Wynne J, et al. Percutaneous balloon valvuloplasty compared with open surgical commissurotomy for mitral stenosis. N Engl J Med. 1994; 331: 961–967.
38. Raymer K, Yang H. Patients with aortic stenosis: cardiac complications in non-cardiac surgery. Can J Anaesth. 1998; 45: 855-859.
39. Torsher LC, Shub C, Rettke SR, Brown DL. Risk of patients with severe aortic stenosis undergoing noncardiac surgery. Am J Cardiol. 1998; 81: 448-452.
40. Thompson RC, Liberthson RR, Lowenstein E. Perioperative anesthetic risk of noncardiac surgery in hypertrophic obstructive cardiomyopathy. JAMA. 1985; 254: 2419–21.
41. Haering JM, Comunale ME, Parker RA, et al. Cardiac risk of noncardiac surgery in patients with asymmetric septal hypertrophy. Anesthesiology. 1996; 85: 254-259.
42. O’Kelly B, Browner WS, Massie B, et al. Ventricular arrhythmias in patients undergoing noncardiac surgery. The Study of Perioperative Ischemia Research Group. JAMA. 1992; 268: 217-21.
43. Mahla E, Rotman B, Rehak P, et al. Perioperative ventricular dysrhythmias in patients with structural heart disease undergoing noncardiac surgery. Anesth Analg. 1998; 86:16-21.
44. Eagle KA, Rihal CS, Mickel MC, et al. Cardiac risk of noncardiac surgery: influence of coronary disease and type of surgery in 3368 operations. CASS Investigators and University of Michigan Heart Care Program. Coronary Artery Surgery Study. Circ J Am Heart Assoc. 1997; 96: 1882-1887.
45. Guidelines and indications for coronary artery bypass graft surgery. A report of the ACC/AHA Task Force on Assessment of Diagnostic and Therapeutic Cardiovascular Procedures (Subcommittee on Coronary Artery Bypass Graft Surgery). J Am Coll Cardiol. 1991; 17: 543-589.
47. Elmore JR, Hallett JW, Jr., Gibbons RJ, et al. Myocardial revascularization before abdominal aortic aneurysmorrhaphy: effect of coronary angioplasty. Mayo Clin Proc. 1993; 68: 637-641.
48. Allen JR, Helling TS, Hartzler GO. Operative procedures not involving the heart after percutaneous transluminal coronary angioplasty. Surg Gynecol Obstet. 1991; 173: 285-288.
49. Gottlieb A, Banoub M, Sprung J, et al. Perioperative cardiovascular morbidity in patients with coronary artery disease undergoing vascular surgery after percutaneous transluminal coronary angioplasty. J Cardiothorac Vasc Anesth. 1998; 12: 501-506.
50. Posner KL, Van Norman GA, Chan V. Adverse cardiac outcomes after noncardiac surgery in patients with prior percutaneous transluminal coronary angioplasty. Anesth Analg. 1999; 89: 553-560.
51. Smith SC, Jr., Dove JT, Jacobs AK, et al. ACC/AHA guidelines of percutaneous coronary interventions (revision of the 1993 PTCA guidelines) — executive summary. A report of the ACC/AHA Task Force on Practice Guidelines (committee to revise the 1993 guidelines for percutaneous transluminal coronary angioplasty). J Am Coll Cardiol. 2001; 37: 2215-2238.
52. Kaluza GL, Joseph J, Lee JR, et al. Catastrophic outcomes of noncardiac surgery soon after coronary stenting. J Am Coll Cardiol. 2000; 35: 1288-1294.
53. Reich DL, Bodian CA, Krol M, et al. Intraoperative hemodynamic predictors of mortality, stroke, and myocardial infarction after coronary artery bypass surgery. Anesth Analg. 1999; 89: 814-822.
54. Hewer I, Drew B, Karp K, Stotts N. The utilization of automated ST segment analysis in the determination of myocardial ischemia. Am Assoc Nurse Anesthetists J. 1997; 65: 351-356.
55. Landesberg G, Luria MH, Cotev S, et al. Importance of long-duration postoperative ST-segment depression in cardiac morbidity after vascular surgery. Lancet 1993; 341: 715-719.
56. Stoschitzky K. Beta-blockers and non-cardiac surgery. J Clin Basic Cardiol. 2001; 4: 21-23.
57. Smulyan H, Weinberg SE, Howanitz PJ. Continuous propranolol infusion following abdominal surgery. JAMA 1982; 247: 2539-2542.
58. Raby KE, Brull SJ, Timimi F, et al. The effect of heart rate control on myocardial ischemia among high-risk patients after vascular surgery. Anesth Analg. 1999; 88: 477-482.
59. Boersma E, Poldermans D, Bax JJ, et al. Predictors of cardiac events after major vascular surgery: Role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. JAMA. 2001; 285: 1865-1873.
60. Wallace A, Layug B, Tateo I, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998; 88: 7-17.
61. Reis SE, Feldman AH. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med. 1997; 336: 1453.
62. Petros JA. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. N Engl J Med.1997; 336: 1452.
63. Litwack R, Gilligan D, DeGruttola V. Beta-Blockade for patients undergoing vascular surgery. N Engl J Med. 2000; 342: 1051-1053.
64. Feldman T, Fusman B, McKinsey JF. Beta-Blockade for patients undergoing vascular surgery. N Engl J Med. 2000; 342: 1051-1052.
65. Poldermans D, Boersma E. Beta-Blockade for patients undergoing vascular surgery. N Engl J Med. 2000; 342: 1052-1053.
66. Pasternack PF, Imparato AM, Baumann FG, et al. The hemodynamics of beta-blockade in patients undergoing abdominal aortic aneurysm repair. Circ J Am Heart Assoc. 1987; 76(suppl 3, pt 2): III-1-7.
67. Auerbach AD, Goldman L. Beta-blockers and reduction cardiac events in noncardiac surgery. JAMA. 2002; 287: 1435-1444.
68. Fleisher LA, Eagle KA, Shaffer T, et al. Perioperative –and long –term mortality rates after major vascular surgery: the relationship to preoperative testing in the medicare population. Anesth Analg. 1999; 89: 849-855.
69. Bayliff CD, Massel DR, Inculet RI, et al. Propranolol for the prevention of postoperative arrhythmias in general thoracic surgery. Ann Thorac Surg. 1999; 67: 182-186.
70. Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001; 141: 148-153.
71. Mangano DT, Goldman L. Preoperative assessment of patients with known or suspected coronary disease. N Engl J Med. 1995; 333:1750-1756.
|