Perioperative beta-blockers: is it still useful?
W-J Flu, J-P van Kuijk, J.J Bax, D. Poldermans
Department of Anaesthesiology, Erasmus Medical Center, Rotterdam, The Netherlands
Department of Cardiology, Leiden University Medical Center, Leiden, The Netherlands
Department of Vascular Surgery, Erasmus Medical Center, Rotterdam, The Netherlands
Introduction
Beta-blockers are established therapeutic agents for patients with hypertension, heart failure and coronary artery disease. There is still considerable debate concerning the protective effect of beta-blocker therapy towards perioperative coronary events, which will be outlined it this review article. The present article provides an overview of leading observational studies, randomized controlled trials, meta-analyses and guidelines assessing perioperative beta-blocker therapy.
Risk stratification in non-cardiac surgery
In the United States, 30 million non-cardiac surgical procedures are performed every year and by 2020 the number of patients eligible for surgery will increase by 25%. The total number of surgical procedures will rise even faster because the frequency of intervention increases dramatically with age. Surgical patients are older and sicker than the general population (27% are older than 65 years versus 11% of the general population) and 40% have atherosclerotic disease risk factors 1. Preoperative risk stratification is of great importance to predict cardiac outcome in patients requiring major non-cardiac surgery. Patients who are at increased risk of postoperative events may benefit from medical treatment or other preoperative interventions. Therefore, identification of these patients is an important goal in preoperative screening strategies. Tools for preoperative screening include resting echocardiography, dobutamine stress echocardiography, biomarkers and cardiac risk scores. Multiple risk indices have been developed over the years 2, 3, however the Lee index (or revised cardiac risk index) is generally considered to be the most widely used index to predict peri-operative cardiac risk 4. In order to optimize the prediction of perioperative mortality in vascular surgery patients, Boersma et al developed a simple risk-index that accounts for significant clinical risk factors and medication use to predict perioperative all-cause mortality. By summing individual scores derived from the given predictors (myocardial infarction, angina pectoris, congestive heart failure, cerebrovascular disease, diabetes mellitus, renal dysfunction and age over 70 years) and with the use of the total risk score, the patient’s probability of perioperative mortality can be estimated 5, 6. Although previous studies emphasize ischemic heart disease as the most important risk factor for perioperative complications, heart failure is known to be as equally important 7.
Cardiac complications in non-cardiac surgery
Correspondence: Don Poldermans, M.D., Ph.D.Department of Vascular Surgery
Erasmus Medical Center, Room H805, ‘s-Gravendijkwal 230, 3015 CE Rotterdam, the Netherlands
E-mail: d.poldermans@erasmusmc.nl
Phone: +31 10 7034613
fax: +31 10 7034957
|
Beta-blocker therapy in non-cardiac surgery:
Mechanism of action
During surgery, adequate hemodynamic control is achieved through sympathetic tone attenuation due to volatile anaesthetics 8. Furthermore, to reduce postoperative morbidity and mortality adequate perioperative medical therapy plays a pivotal role and should at least contain a beta-blocker in high-risk patients. Beta-blockers are established therapeutic agents for patients with hypertension, heart failure and coronary artery disease. In the non-surgical setting beta-blockers are widely used for the prevention and treatment of ischemic heart disease and heart failure, all major determinants of adverse postoperative outcome. Beta-blockers are known to exert: 1) anti-arrhythmic effects, 2) anti-inflammatory effects, 3) antirenin-angiotensin properties and 4) a shift in energy metabolism 16-18. Surgical procedures are associated with perioperative tachycardia and increased myocardial contractility leading to an increased oxygen demand 13. Beta-blockers have shown to reduce the myocardial oxygen demand by reducing heart rate, systolic pressure and ventricular contractile force. Furthermore, Beta-blockers promote coronary plaque stability by reducing mechanical and shear stresses and their anti-inflammatory properties have a beneficial effect towards coronary plaque stabilization as well 19, 20.
An overview of the literature
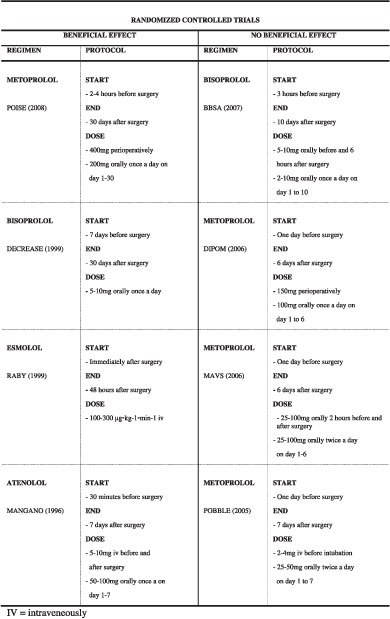
metoprolol or placebo twice a day. Treatment started 2 hours prior to surgery and continued until hospital discharge or 5 days after surgery. The Diabetic Postoperative Mortality and Morbidity (DIPOM) trial was published in 2006 and evaluated perioperative treatment with metoprolol (50mg prior to surgery, 100 mg day of surgery and 100mg postoperatively from day 1 to 6) in 921 diabetic patients. They concluded that metoprolol did not significantly reduced 30-day cardiac morbidity and mortality (21% versus 20%; P=0.66) in patients with diabetes 23. Perioperative treatment with Bisoprolol was evaluated in elderly patients undergoing surgery with neuraxial blockade in the Swiss Beta Blocker in Spinal Anesthesia (BBSA) trial 24. In this underpowered study, in which 226 patients were included, bisoprolol did not exert beneficial effects towards cardiovascular outcome, possible due to varying cardiac risk profile of the patients included.
Evidence of beneficial effects of perioperative beta-blocker treatment to reduce perioperative cardiovascular complications is provided by several observational studies 25, 26. 27 28, and more importantly by several randomized controlled trials. A randomized controlled trial conducted by Mangano et al was published in 1996 and included 200 patients with known or suspected coronary artery disease undergoing high-risk non-cardiac surgery 29. Included patients received either atenolol (50mg or 100mg) or placebo before the induction of anaesthesia and immediately after surgery. Atenolol treatment was continued up to 2 years after surgery in most patients and atenolol use was associated with significantly lower mortality rates at 6 months after discharge (0% versus 8%; P=0.005), and after 2 years (10% versus 21%; P=0.019). Beneficial effects of beta-blocker treatment immediately after surgery was demonstrated in 1999 in a study conducted by Raby et al in 1999. They included 26 patients with preoperative ischemia detected by Holter monitoring undergoing major vascular surgery, randomized to receive esmolol (100-300 μg◦kg-1◦min-1 intravenously for 48 hours after surgery) or placebo postoperatively 30. This study was the first to demonstrate that strict heart rate control after surgery, to 20% below the ischemic threshold, markedly reduced the occurrence of postoperative ischemia (placeco: 73% versus esmolol 33%, p<0.05).
DECREASE-I and POISE trial
In 1999, the Poldermans group published the first Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography (DECREASE-I) trial. Primary objective was to evaluate the effect of perioperative bisoprolol treatment on occurrence of nonfatal myocardial infarction and cardiac death within 30 days after major vascular surgery 31. In total 112 cardiac high-risk patients with evidence of myocardial ischemia during preoperative dobutamine stress-echocardiography were included. Around half the patients received bisoprolol (2.5-10mg) carefully titrated to obtain a perioperative heart rate between 60-65 beats per minute.
|
Treatment was started at an average of 37 days prior to surgery and treatment continued at least 30 days after treatment. In patients treated with bisoprolol a reduction in the incidence of perioperative cardiovascular death and myocardial infarction from 34% to 3.4% (P<0.001), was demonstrated 31 compared to placebo. Although these results were promising, the PeriOperative ISchemic Evaluation (POISE) trial, published in 2008, caused great discussion regarding side-effects caused by perioperative treatment with beta-blokcers. The POISE trial included 8351 patients either to receive placebo or treatment with metoprolol succinate using the following treatment protocol: 1) 100mg was given 2-4 hours prior to surgery 2) another 100mg within 6 hours and followed by 3) another 200mg 12-18 hours post surgery if permitted by heart rate and blood pressure. The primary endpoint (cardiac death, myocardial infarction or cardiac arrest) was reduced in the metoprolol group, compared with placebo (5.8% vs. 6.9%, hazard ratio 0.83, 95% CI 0.70-0.99, p=0.04). However, the 30% decrease of non-fatal myocardial infarction (3.6 versus 5.1%, p=0.0008) was accompanied by a 33% increase in total mortality (3.1% versus 2.3%, p=0.03) and a twofold increase risk in stroke (1.0 versus 0.5%, p=0.005) 32. The above mentioned randomized controlled trial are summarized in Table 1.
 |
Putting the literature in perspective
The most important clinical trials to evaluate the effect of perioperative beta-blocker treatment have brought forward conflicting results regarding its efficacy. Treatment with bisoprolol was associated with better results compared to metoprolol or atenolol. The type of beta-blocker used, therefore, might influence the effectiveness of beta-blocker therapy. In comparison with other beta 1-adrenoceptor antagonists (e.g. atenolol, metoprolol), bisoprolol proved to be the compound with the highest beta-1 selectivity in all in vitro and in vivo experiments and in all animal species investigated 33-36. Negative inotropic and chonotropic effects derived from highly selective beta-1 blockade might exert most beneficial perioperative effects towards cardiovascular outcome. Another important factor might give an explanation for these conflicting results may be the variation in treatment protocols of the conducted studies. We have discussed four randomized trials in which the beneficial effect of perioperative beta-blocker use was not shown (POBBLE, MAVS, DIPOM and BBSA). In these trial, beta-blocker treatment was started one day prior to surgery or at the day of surgery. In comparison, in the DECREASE-I trial the mean time between initiation of beta-blocker treatment and surgery was 37 days and in the DECREASE-I trial the largest effect of perioperative beta-blocker treatment was demonstrated 31. In addition, the administrated dosage of beta-blocker were different in the above mentioned randomized studies. For instance, in the DECREASE-I trial, vascular surgery patients were treated with low-dose bisoprolol, between 5-10mg once daily. The incidence of stroke in the DECREASE-1 trials was 0,4%, comparable with placebo, while maintaining a significant reduction in cardiac death and nonfatal myocardial infarction from 34% in the standard-care group to 3,4% in the bisoprolol treated group in the first DECREASE-1 trial 31, 37. In comparison, treatment protocol of the POISE trial showed that metoprolol succinate could have been administered on the first day of surgery at a dose up to 400 mg on the day of surgery which resembles the maximum daily therapeutic dose. However, in heart failure patients much lower starting doses are recommended. In patients with NYHA Class II heart failure 12.5 to 25 mg daily is started for two weeks and for hypertension the initial dose is 25 to 100 mg, usually increased at weekly intervals.
As indicated by the randomized controlled trials conducted by Mangano et al and Raby et al and further demonstrated in the Decrease-I trial, tight heart rate control will maximize the benefit a patients derives from beta-blocker treatment. However, one should avoid to over treat the patient. The most important side effects, which are to be expected with beta-blocker treatment, are bradycardia and hypotension, which usually occur dose-dependently. As already mentioned, the POISE-trial showed that metoprolol succinate treatment did lower the incidence of myocardial infarction by more than a quarter (5.7% to 4.2%). However this benefit was outweighed by an increased incidence of stroke and death 32. Post-hoc analyzing demonstrated that stroke was associated with perioperative bradycardia, hypotension, and bleeding complications. Post-hoc analysis also showed that hypotension had the largest population-attributable risk for death and stroke. Importantly, hypotension could be related to the use of a high dose of metoprolol without dose titration in the POISE-trial. Therefore, analysing the safety and tolerability of beta-blockers is as important as assessing the beneficial effects of beta-blockers regarding efficacy. Titration according to tolerance is of utmost importance to obtain tight heart rate control and prevent adverse side effects such as hypotension and bradycardia. The value of adequate heart rate control in improving cardiovascular outcome is not only confirmed in a recent large meta-analysis 38, the latest 2007 ACC/AA guidelines on perioperative care strongly recommend achieving a heart rate between 65-70 beats per minute 39.
|
In 2001 Shammash et al evaluated the influence of perioperative beta-blocker withdrawal towards postoperative mortality of vascular surgical patients. They have suggested that discontinuation of beta-blocker therapy immediately after surgery might increase the risk of postoperative cardiovascular mortality 40. This notion is supported by a study conducted by Hoeks et al, published in 2007, who found that withdrawal of beta-blockers early after surgery is associated with a higher one-year mortality compared to continuous beta-blocker therapy. This study has highlighted the importance of continue beta-blocker therapy in the perioperative period 41. Therefore, the duration of beta-blocker therapy might be a factor of influence on the mixed results provided by the randomized trial which are discussed in this review article. In the DECREASE-I trial patients were treated at least 30 day after surgery and in the study conducted by Mangano et al most patients received atenolol treatment up to two years after surgery. Atenolol treatment showed to reduce mortality and the occurrence of cardiovascular events up to two years after surgery 29. Perioperative beta-blocker withdrawal might result in a “rebound” effect causing an increase of arterial blood pressure, heart rate and plasma noradrenalin concentrations 42. Esmolol is an ultra-short-acting cardioselective, beta-adrenergic blocking agent with a rapid onset of around 60 seconds and short duration of action between 10 to 20 minutes 43-45. Intra-operative infusion with esmolol might be effective to prevent intra-operative tachycardia and reduce intra-operative left ventricular contractile force. The short acting character of esmolol and continuous hemodynamic monitoring during surgery can limit adverse side effects, such as hypotension or bradycardia. In addition, the long-term beneficial effects of beta-blocker therapy might be explained by a decrease of progress of coronary atherosclerosis 46. In contrast to the instant effect on heart rate control, the effect of beta-blockers on plaque stabilization may therefore be achieved only after prolonged treatment.
Non-cardiac surgery: with or without beta-blockers?
DECREASE-I trial included high-risk cardiac patients. In 2008, a meta-analysis was published in the Lancet addressing the effects of perioperative beta-blocker treatment in more than 12.000 patients. The main result was that beta-blocker treatment resulted in 16 fewer non-fatal myocardial infarctions per 1000 patients, but at the expense of three non-fatal disabling strokes and possibly three fatal cardiac or non-cardiac complications 48. However, around two third of the patients were derived from the POISE trial, and as the author acknowledge, these patients had the greatest weight for the results. A comment Boersma and Poldermans was published in the same edition of the Lancet, in which they conclude that the general mechanism that might explain the excess cerebral complications is unknown and additional hemodynamic data are needed. They address that these hemodynamic data will be the key for updates of treatment guidelines 49. Multiple studies have provided evidence that there is an under-use of beta-blockers in surgery patient 1) even when patients are considered to be at high-risk for cardiovascular events, 2) despite an increase in beta-blocker prescription worldwide 41, 50 and the 3) despite the fact that the ACC/AHA 2006 guidelines (update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy) 51 advocate perioperative beta-blocker use.
Conclusions
We conclude that long acting beta-blockers, such as bisoprolol, seem to be the agent of preference to reduce adverse perioperative events and enhance postoperative survival. Low dose treatment, initiated al least a month prior to surgery, and up-titration according to tolerance and obtain heart rates between 65 and 70 beats per minute could demonstrate maximal protection without over treating the patients. Furthermore, prolonged treatment after surgery is favoured to maximize the anti-inflammatory properties of beta-blocker therapy with beneficial effects on coronary plaque stabilization. Intra-operatively, the ultra short beta-blocker esmolol, administered via a continuous infusion, could be considered as well to maximally prevent the occurrence of adverse myocardial events. A randomized controlled trial, addressing pre- and postoperative treatment with low-dose bisoprolol and intra-operative treatment with esmolol could demonstrate a maximal protective effect derived from beta-blocker treatment. Patients with at least two revised cardiac risk factors (ischemic heart disease, congestive heart failure, cerebrovascular disease, diabetes mellitus, renal dysfunction, age over 70 years and high-risk surgery) should be included to assess maximal risk reduction and provide us with the final answer to the question: “perioperative beta-blockers: is it still useful”.
|
References
1. Mangano DT. Perioperative medicine: NHLBI working group deliberations and recommendations. J Cardiothorac Vasc Anesth 2004;18(1):1-6.
2. Detsky AS, Abrams HB, Forbath N, Scott JG, Hilliard JR. Cardiac assessment for patients undergoing noncardiac surgery. A multifactorial clinical risk index. Arch Intern Med 1986;146(11):2131-4.
3. Goldman L, Caldera DL, Nussbaum SR, Southwick FS, Krogstad D, Murray B, et al. Multifactorial index of cardiac risk in noncardiac surgical procedures. N Engl J Med 1977;297(16):845-50.
4. Lee TH, Marcantonio ER, Mangione CM, Thomas EJ, Polanczyk CA, Cook EF, et al. Derivation and prospective validation of a simple index for prediction of cardiac risk of major noncardiac surgery. Circulation 1999;100(10):1043-9.
5. Boersma E, Kertai MD, Schouten O, Bax JJ, Noordzij P, Steyerberg EW, et al. Perioperative cardiovascular mortality in noncardiac surgery: validation of the Lee cardiac risk index. Am J Med 2005;118(10):1134-41.
6. Kertai MD, Boersma E, Klein J, van Sambeek M, Schouten O, van Urk H, et al. Optimizing the prediction of perioperative mortality in vascular surgery by using a customized probability model. Arch Intern Med 2005;165(8):898-904.
7. Hernandez AF, Whellan DJ, Stroud S, Sun JL, O'Connor CM, Jollis JG. Outcomes in heart failure patients after major noncardiac surgery. J Am Coll Cardiol 2004;44(7):1446-53.
8. Mangano DT. Perioperative cardiac morbidity. Anesthesiology 1990;72(1):153-84.
9. Mangano DT. Adverse outcomes after surgery in the year 2001--a continuing odyssey. Anesthesiology 1998;88(3):561-4.
10. Ouyang P, Gerstenblith G, Furman WR, Golueke PJ, Gottlieb SO. Frequency and significance of early postoperative silent myocardial ischemia in patients having peripheral vascular surgery. Am J Cardiol 1989;64(18):1113-6.
11. Feringa HH, Karagiannis SE, Vidakovic R, Elhendy A, ten Cate FJ, Noordzij PG, et al. The prevalence and prognosis of unrecognized myocardial infarction and silent myocardial ischemia in patients undergoing major vascular surgery. Coron Artery Dis 2007;18(7):571-6.
12. Ardehali A, Ports TA. Myocardial oxygen supply and demand. Chest 1990;98(3):699-705.
13. Priebe HJ. Perioperative myocardial infarction--aetiology and prevention. Br J Anaesth 2005;95(1):3-19.
14. Cohn JN, Ferrari R, Sharpe N. Cardiac remodeling--concepts and clinical implications: a consensus paper from an international forum on cardiac remodeling. Behalf of an International Forum on Cardiac Remodeling. J Am Coll Cardiol 2000;35(3):569-82.
15. Schrier RW, Ecder T. Gibbs memorial lecture. Unifying hypothesis of body fluid volume regulation: implications for cardiac failure and cirrhosis. Mt Sinai J Med 2001;68(6):350-61.
16. Yeager MP, Fillinger MP, Hettleman BD, Hartman GS. Perioperative beta-blockade and late cardiac outcomes: a complementary hypothesis. J Cardiothorac Vasc Anesth 2005;19(2):237-41.
17. Stanley WC, Lopaschuk GD, Hall JL, McCormack JG. Regulation of myocardial carbohydrate metabolism under normal and ischaemic conditions. Potential for pharmacological interventions. Cardiovasc Res 1997;33(2):243-57.
18. Cruickshank JM. Beta-blockers continue to surprise us. Eur Heart J 2000;21(5):354-64.
19. Dawood MM, Gutpa DK, Southern J, Walia A, Atkinson JB, Eagle KA. Pathology of fatal perioperative myocardial infarction: implications regarding pathophysiology and prevention. Int J Cardiol 1996;57(1):37-44.
20. Warltier DC, Pagel PS, Kersten JR. Approaches to the prevention of perioperative myocardial ischemia. Anesthesiology 2000;92(1):253-9.
21. Brady AR, Gibbs JS, Greenhalgh RM, Powell JT, Sydes MR. Perioperative beta-blockade (POBBLE) for patients undergoing infrarenal vascular surgery: results of a randomized double-blind controlled trial. J Vasc Surg 2005;41(4):602-9.
22. Yang H, Raymer K, Butler R, Parlow J, Roberts R. The effects of perioperative beta-blockade: results of the Metoprolol after Vascular Surgery (MaVS) study, a randomized controlled trial. Am Heart J 2006;152(5):983-90.
23. Juul AB, Wetterslev J, Gluud C, Kofoed-Enevoldsen A, Jensen G, Callesen T, et al. Effect of perioperative beta blockade in patients with diabetes undergoing major non-cardiac surgery: randomised placebo controlled, blinded multicentre trial. Bmj 2006;332(7556):1482.
24. Zaugg M, Bestmann L, Wacker J, Lucchinetti E, Boltres A, Schulz C, et al. Adrenergic receptor genotype but not perioperative bisoprolol therapy may determine cardiovascular outcome in at-risk patients undergoing surgery with spinal block: the Swiss Beta Blocker in Spinal Anesthesia (BBSA) study: a double-blinded, placebo-controlled, multicenter trial with 1-year follow-up. Anesthesiology 2007;107(1):33-44.
25. Wallace A, Layug B, Tateo I, Li J, Hollenberg M, Browner W, et al. Prophylactic atenolol reduces postoperative myocardial ischemia. McSPI Research Group. Anesthesiology 1998;88(1):7-17.
26. Redelmeier D, Scales D, Kopp A. Beta blockers for elective surgery in elderly patients: population based, retrospective cohort study. Bmj 2005;331(7522):932.
27. Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med 2005;353(4):349-61.
28. Feringa HH, Bax JJ, Boersma E, Kertai MD, Meij SH, Galal W, et al. High-dose beta-blockers and tight heart rate control reduce myocardial ischemia and troponin T release in vascular surgery patients. Circulation 2006;114(1 Suppl):I344-9
29. Mangano DT, Layug EL, Wallace A, Tateo I. Effect of atenolol on mortality and cardiovascular morbidity after noncardiac surgery. Multicenter Study of Perioperative Ischemia Research Group. N Engl J Med 1996;335(23):1713-20.
30. Raby KE, Brull SJ, Timimi F, Akhtar S, Rosenbaum S, Naimi C, et al. The Effect of Heart Rate Control on Myocardial Ischemia Among High-Risk Patients After Vascular Surgery. Anesth Analg 1999;88(3):477-482.
31. Poldermans D, Boersma E, Bax JJ, Thomson IR, van de Ven LL, Blankensteijn JD, et al. The effect of bisoprolol on perioperative mortality and myocardial infarction in high-risk patients undergoing vascular surgery. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study Group. N Engl J Med 1999;341(24):1789-94.
32. Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, et al. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomised controlled trial. Lancet 2008;371(9627):1839-47.
33. Maack C, Tyroller S, Schnabel P, Cremers B, Dabew E, Sudkamp M, et al. Characterization of beta(1)-selectivity, adrenoceptor-G(s)-protein interaction and inverse agonism of nebivolol in human myocardium. Br J Pharmacol 2001;132(8):1817-26.
34. Schliep HJ, Schulze E, Harting J, Haeusler G. Antagonistic effects of bisoprolol on several beta-adrenoceptor-mediated actions in anaesthetized cats. Eur J Pharmacol 1986;123(2):253-61.
35. Smith C, Teitler M. Beta-blocker selectivity at cloned human beta 1- and beta 2-adrenergic receptors. Cardiovasc Drugs Ther 1999;13(2):123-6.
36. Wellstein A, Palm D, Belz GG. Affinity and selectivity of beta-adrenoceptor antagonists in vitro. J Cardiovasc Pharmacol 1986;8 Suppl 11:S36-40.
37. Fleisher LA, Poldermans D. Perioperative beta blockade: where do we go from here? Lancet 2008;371(9627):1813-4.
38. Beattie WS, Wijeysundera DN, Karkouti K, McCluskey S, Tait G. Does tight heart rate control improve beta-blocker efficacy? An updated analysis of the noncardiac surgical randomized trials. Anesth Analg 2008;106(4):1039-48, table of contents.
39. Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2007 Guidelines on Perioperative Cardiovascular Evaluation and Care for Noncardiac Surgery: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery): Developed in Collaboration With the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, Society for Vascular Medicine and Biology, and Society for Vascular Surgery. Circulation 2007;116(17):1971-96.
40. Shammash JB, Trost JC, Gold JM, Berlin JA, Golden MA, Kimmel SE. Perioperative beta-blocker withdrawal and mortality in vascular surgical patients. Am Heart J 2001;141(1):148-53.
41. Hoeks SE, Scholte Op Reimer WJ, van Urk H, Jorning PJ, Boersma E, Simoons ML, et al. Increase of 1-year Mortality After Perioperative Beta-blocker Withdrawal in Endovascular and Vascular Surgery Patients. Eur J Vasc Endovasc Surg 2007;33(1):13-9.
42. Maling TJ, Dollery CT. Changes in blood pressure, heart rate, and plasma noradrenaline concentration after sudden withdrawal of propranolol. Br Med J 1979;2(6186):366-7.
43. Lowenthal DT, Porter RS, Saris SD, Bies CM, Slegowski MB, Staudacher A. Clinical pharmacology, pharmacodynamics and interactions with esmolol. Am J Cardiol 1985;56(11):14F-18F.
44. Reynolds RD, Gorczynski RJ, Quon CY. Pharmacology and pharmacokinetics of esmolol. J Clin Pharmacol 1986;26 Suppl A:A3-A14.
45. Gray RJ. Managing critically ill patients with esmolol. An ultra short-acting beta-adrenergic blocker. Chest 1988;93(2):398-403.
46. Sipahi I, Tuzcu EM, Wolski KE, Nicholls SJ, Schoenhagen P, Hu B, et al. Beta-blockers and progression of coronary atherosclerosis: pooled analysis of 4 intravascular ultrasonography trials. Ann Intern Med 2007;147(1):10-8.
47. Boersma E, Poldermans D, Bax JJ, Steyerberg EW, Thomson IR, Banga JD, et al. Predictors of cardiac events after major vascular surgery: Role of clinical characteristics, dobutamine echocardiography, and beta-blocker therapy. Jama 2001;285(14):1865-73.
48. Bangalore S, Wetterslev J, Pranesh S, Sawhney S, Gluud C, Messerli FH. Perioperative beta blockers in patients having non-cardiac surgery: a meta-analysis. Lancet 2008;372(9654):1962-76.
49. Boersma E, Poldermans D. Beta blockers in non-cardiac surgery: haemodynamic data needed. Lancet 2008;372(9654):1930-2.
50. Siddiqui AK, Ahmed S, Delbeau H, Conner D, Mattana J. Lack of physician concordance with guidelines on the perioperative use of beta-blockers. Arch Intern Med 2004;164(6):664-7.
51. Fleisher LA, Beckman JA, Brown KA, Calkins H, Chaikof E, Fleischmann KE, et al. ACC/AHA 2006 guideline update on perioperative cardiovascular evaluation for noncardiac surgery: focused update on perioperative beta-blocker therapy: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2002 Guidelines on Perioperative Cardiovascular Evaluation for Noncardiac Surgery) developed in collaboration with the American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Rhythm Society, Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society for Vascular Medicine and Biology. J Am Coll Cardiol 2006;47(11):2343-55.
|