Beta-blockers and heart failure Dr. John M Cruickshank Independent Cardiovascular consultant Oxonian Cardiovascular consultancy, Long Melford, Great Britain |
Introduction
 |
||||
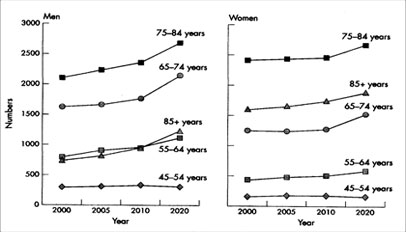
|
a) Systolic HF (low ejection fraction - EF)
Pathophysiological mechanisms underlying systolic HF have changed over the years. Nowadays coronary artery disease (CAD) is responsible for about 65% of systolic HF (6). However, in the elderly, 75% have a past history of hypertension (7). Obesity is now recognised as a risk factor, doubling the risk of developing systolic HF (8). In idiopathic dilated cardiomyopathy (DCM) stimulating autoantibodies to the beta-1 receptor are detected in abut one third of cases, and are associated with a 3-fold increase in mortality(9). Such stimulating autoantibodies induce cardiomyocyte apoptosis (10) and can be antagonised by beta-1 blockade (bisoprolol) (11).
The prognosis for systolic HF patients newly admitted to hospital is poor, with one-year survival only 57%, falling to 27% after five years (12). The worst prognosis occurs in patients who are old, low systolic blood pressure, high respiratory rate or poor renal function (13). Southern Asians are more likely than Caucasians to be admitted to hospital with systolic HF but have a similar prognosis (14). Even asymptomatic patients with a low left ventricular ejection fraction (EF) have a poor prognosis (15).
b) Diastolic HF.
About 50% of HF cases have so-called diastolic HF (10). Because diastolic HF is usually associated with some degree of regional systolic dysfunction some prefer to term it HF with a normal EF. Typical characteristics of patients with diastolic or systolic HF are shown in table 1. The prognosis of diastolic HF is either similar (16), or better (17) than, systolic HF.
The classic case of diastolic HF would be an elderly female with a history of systolic hypertension, concentric left-ventricular hypertrophy (LVH) with a small left ventricular cavity (18). Such patients have a stiff left ventricle with increased end-diastolic and pulmonary pressure during exercise (19), resulting in effort intolerance, increased sypmpathetic nerve activity and raised brain naturetic peptide (20).
Beta-blockers and mortality in systolic heart failure
a) History
Correspondence: Dr. Dr. John M. Cruickshank, Independent Cardiovascular consultant, Oxonian Cardiovascular consultancy, Long Melford, Great Britain .
Email: johndtl@aol.com
|
However chronic dosing of beta-blockers to patients with systolic HF results in beneficial haemodynamic changes resulting in significant increases in exercise capacity (mean 25%). EF (mean 39%) and cardiac index (mean 12%), and a small increase in arterial pressure (mean 3%) (23). Two studies indicated that such apparently beneficial haemodynamic changes were actually linked to an improved clinical outcome. In the BHAT post infarction study (24) comparing placebo and propranolol, patients with a history of HF did better on propranolol in terms of fewer episodes of HF(vs placebo) compared to those without a history of HF. A similar result occurred with timolol vs placebo in the Norwegian post infarction study(25).
In a placebo-controlled study involving patients with idiopathic dilated cardiomyopathy (25) there were significantly fewer primary end-points with metoprolol; in particular only 2 patients on metoprolol deteriorated to the extent of requiring cardiac transplantation compared to 19 receiving placebo.
The scene was thus set for large, randomised, controlled studies to prove or disprove that beta-blockers would benefit patients with systolic heart failure.
b) Large randomised placebo-controlled studies involving beta-blockers in systolic heart failure (with pre defined end-point of all-cause death); all patients on background ACE-inhibitor therapy
The results are shown in Table 2. It is clear that those beta-blockers without intrinsic sympathomemetic activity (!SA) were effective in reducing all-cause mortality by 34-5%(27-9). A marked reduction in sudden death (42%) was particularly notable with bisoprolol in the CIBIS II trial (27). The common property of the three beta-blockers involved (bisoprolol, metoprolol and carvedilol) was beta-1 blockade; thus beta-1 blockade is the active ingredient responsible for reducing all-cause death.
In contrast, the negative results (30-32) were associated with beta-blockers containing ISA, involving either the beta-1 receptor (xamoterol), (30) the beta-1 and beta-2 receptors (bucindolol) (31) or the beta-2 and beta-3 receptors (nebivolol) (32).
c) Do all systolic heart failure patients benefit from beta-1 blockade?
In CIBIS II (27) patients whose HF was due to ischaemia gained particular benefit. In MERIT-HF (28) elderly (> 65 years) patients obtained at least the same benefit as younger patients in terms of lives saved and hospitalization avoided (33). In MERIT-HR (28) black patients benefitted in a similar fashion to white patients (34). Patients with atrial fibrillation benefit from beta-blockade, with or without digoxin, to a tune of 42% reduction in mortality (34a).
d) Why does ISA markedly diminish the efficacy of beta-blockers in the treatment of systolic heart failure?
Firstly the fall in heart rate is less with beta-blockers with ISA (thus the work of the heart is reduced less). Xamoterol actually increased resting heart-rate (35). The fall in heart rate with bucindolol and nebivolol was only 8-9 bpm (31-2) compared to 13-14 bpm with the non-ISA beta-blockers (28-9).
Bucindolol displays about 25% ISA (36) acting mainly through the beta-1 receptor (37. ) But this beta-blocker also appears to effect a marked sympatholytic action (38) which appears to be potentially harmful in severe systolic HF. In the BEST study (31) high base-line norepineprine levels in the placebo group were associated with an increased mortality: by contrast buccindolol-induced falls in nepinephrine were also associated with an increased mortality (probably by removing the sustaining norepinephrine support in severe HF). This harmful effect is reminiscent of the clonidine-like moxonidine action in HF. This alpha-2 and I1 receptor-agonist acts centrally to reduce norepinephrine outflow (39), which results in an increased mortality in moderate to severe HF.
Nebivolol possesses ISA which acts via the beta-3 receptors in the vascular endothelium (40) and the heart (41), resulting in nitric oxide (NO) release. NO causes potentially advantageous vasodilation and vascular protection but its effects upon the heart are more problematical. In the normal heart beta-3 stimulation is potentially protective in contrast to the failing heart where it is possibly harmful (42). In the failing heart there is beta-3 receptor up-regulation (42) and beta-3 stimulation has a negative inotropic effect leading to myocardial dysfunction (42-3). Indeed in the post-myocardial period L-arginine (substrate for nitric oxide synthase) increased mortality vs placebo (44).
e) Other placebo-controlled heart-failure studies involving beta-blockers
Table 1: Characteristics of patients with diastolic and systolic heart failure
Characteristic |
Diastolic Heart Failure |
Systolic Heart Failure |
Age |
Frequently elderly |
All ages, typically 50-70 yr |
Sex |
Frequently female |
More often male |
Left ventricular ejection fraction |
Preserved or normal, approximately 40% or higher |
Depressed, approximately 40% or lower |
Left ventricular cavity size |
Usually normal, often with concentric left ventricular hypertrophy |
Usually dilated |
Left /ventricular hypertrophy on electrocardiography |
Usually present |
Sometimes present |
Chest radiography |
With or without cardiomegaly |
Congestion and cardiomegaly |
Gallop rhythm present |
Fourth heart sound |
Third heart sound |
Coexisting conditions |
|
|
Hypertension |
+++ |
++ |
Diabetes |
+++ |
++ |
Previous myocardial infarction |
+ |
+++ |
Obesity |
+++ |
+ |
Chronic lung disease |
++ |
0 |
Sleep apnoea |
++ |
++ |
Long-term dialysis |
++ |
0 |
Atrial fibrillation |
+(usually paroxysmal) |
+(usually persistent) |
|
|
|
Table 2: Placebo-controlled heart-failure trials involving beta-blockers – all cause death
|
Study |
BB |
ISA |
HF severity |
Number of patients |
All-cause mortality |
Statist |
Positive Trials |
CIBIS II (26) |
Bisoprolol (high B-1 selective) |
No |
Mod - severe |
2649 |
↓34% |
Yes |
MERIT (27) |
Metoprolol succinate (mod beta-1 selective) |
No |
Mild - moderate |
3991 |
↓ 34% |
Yes |
|
COPERNICUS (28) |
Carvedilol (non-selective + alpha-blocker) |
No |
Severe |
2289 |
↓ 35% |
Yes |
|
Negative Trials |
Xamoterol(29) |
Xamoterol (beta-1 selective) |
43% beta-1 ISA |
Mod-severe |
516 |
↑ 249% |
(yes) |
BEST (30) |
Bucindolol (weak alpha-blocker + non selective) |
25% ISA |
Mod - severe |
2708 |
↓ 10% |
No |
|
SENIORS(31) |
Nebivolol (beta-1 selective) |
Both beta-2 and beta-3 ISA |
Mod-severe |
2128 |
↓ 12% |
No |
|
The CARPRICORN study (46) involved post-myocardial function patients with impaired left ventricular function. There was a significant 23% reduction in all-cause death on carvedilol vs placebo.
An interesting study in children and adolescents (less than 18 years old) with heart failure (47) showed that carvedilol did not differ from placebo, though all trends, including all-cause mortality, favoured the beta-blocker.
f) Comparative studies (non-placebo) in heart failure involving beta-blockers
i) COMET Study(48) – carvedilol vs metoprolol
This double-blind, randomised study comparing metoprolol tartrate and carvedilol in 3029 moderate/severe heart failure cases showed that over a six year follow-up period all-cause mortality was significantly lower (17%) on carvediolol.
The result was met with a severe criticism (49-50) the nub of which revolved around dose and plasma half-lives. In the original MERIT-HF study (28) metoprolol succinate was given as a controlled release/extended release formula (metoprolol CR/XL) which was dosed up to 200 mg daily, lowering heart-rate by 14 bpm and reducing all-cause mortality by 34% (same as the 34-5% reduction with bisoprolol (27) and carvedilol (29) vs placebo). In the #COMET study (48) short-acting metoprolol tartrate was used, dosed up to 100 mg daily and lowering heart-rate by 11 bpm. Thus the poor result for metoprolol in COMET could easily be explained by too-low a dose rendering inadequate beta-1 blockade over 24 hours.
ii) CIBIS –III study(51) – first-line bisoprolol vs first-line enalapril
This study addressed the issue of whether a beta-blocker given first-line would be “not inferior” to first-line ACE-inhibition, in patients with mild-moderate HF; the 2 drugs were combined after 6 months. First-line bisoprolol proved “not-inferior” to first-line enalapril in reducing the primary end-point of all-cause mortality or hospitalization and was at least as well tolerated. In pre-specified sub-groups analysis patients with an EF of less than 28% fared significantly better on bisoprolol than enalapril. Sudden death was 46% less common after one year in the first-line beta-blocker group (52) – Figure 3.
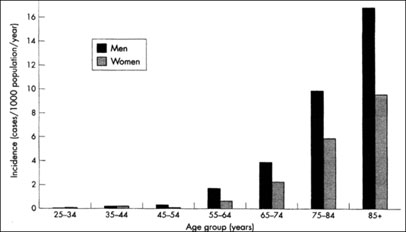
4. Early Identification of Asian Indians at High Risk for CAD
Those who have >2 risk factors or metabolic syndrome are considered high risk individuals. Most but not all experts agree that metabolic syndrome identifies individuals who are generally at low short-term (10-year) risk for CVD but at very high lifetime risk of CVD and diabetes (type 2), but more importantly, respond well to intensive life style modification.22 It is important to recognize that the US National Cholesterol Education Program (NCEP) criteria may under-estimate the prevalence of metabolic syndrome by up to 50%.22 The South Asian Modified NCEP criteria for metabolic syndrome substitutes the waist circumference cut points shown above (>90 cm for men and >80 cm for women) in the NCEP criteria and is more appropriate for Asian Indians (Table 3).14,22
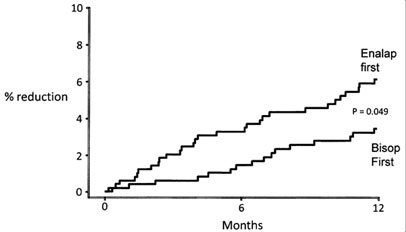 |
| Figure 3 = CIBIS III – prevention of sudden death (significant 46% reduction) with first-line bisoprolol vs first-line enalapril (52) |
Mechanism of action of beta-blockers in moderate-severe systolic heart failure
These have already been discussed (53), and possible mechanisms involve –
a) Bradycardia, leading to i) reduction in cardiac work and thus a reduction in oxygen requirement, and ii) prolonged diastolic coronary-filling-time.
b) Anti-ventricular dysrhythmia activity.
c) Up-regulation of cardiac beta-1 receptors
d) Inhibition of the renin/angiotensin system.
e) Increase in brain naturetic peptide (BNP).
f) Beneficial effects on left-ventricular remodelling (54), resulting in improved left-ventricular volumes and function and improved EF.
g) Antagonism of stimulatory beta-1 receptor auto-antibodies.
h) Inhibition of catecholamine-induced necrosis/apoptosis/inflammation.
|
The last (h) highly relevant, important mechanism of catecholamine-induced cardiac damage, is particularly interesting when both the beta-1 and the beta-2 receptors are considered. Stimulation of the beta-1 receptor induces myocardial myocyte apoptosis/necrosis via a C-AMP-dependent process; whereas stimulation of the beta-2 receptor inhibits myocardial apoptosis/necrosis via a Gi- coupled pathway (55). The implication is that beta-2 blockade would be potentially harmful - Figure 4. Certainly beta-1 blockade in humans has been shown to prevent cardiac necrosis (56) and may be acting by stabilising the ryanodine receptor on cardiac sarcoplasmic reticulum thus preventing excessive calcium release and related poor myocyte function and increased risk of ventricular fibrillation (57). Specific beta-2 blockade (ICI 118,551) has been observed to induce a marked negative inotropic effect in isolated myocytes from failing human hearts (58). This action is direct and is quite independent of inhibiting external beta-2 stimulation.
The clinical implication of these observations are illustrated in the next section.
End-stage systolic heart failure and specific beta-2 stimulation plus specific beta-1 blockade
End-stage HF, in spite of optimal therapy with positive inotropes, ACE-inhibitors, angiotensin-1 receptor antagonists and beta-blockade, requires urgent cardiac transplantation. However transplantation is not always an ideal solution, with 15-20% of recipients dying within 1 year of the operation and only 15% survive after 20 years (59). The quality of survival is usually markedly impaired due to the requirement of immunsuppression e.g. poor renal function, severe coronary artery disease and high risk of skin cancers and lymphoma.
However there is now new hope for such patients, which involves pharmocothoapy rather than transplantation (60). Fifteen end-stage HF cases (non-ischaemic cardiomyopathy), aged 15-56 years, and with a mean EF of only 12%, were surviving on inotropic therapy. All underwent implantation of a left ventricular assist device, and were prescribed lisinopril, losartan, spironolactone and carvediolol. When cardiac enlargement regressed, carvedilol was exchanged for highly beta-1 selective bisoprolol, followed by the beta-2 stimulant clenbuterol (non-selective carvedilol would have blocked its action on beta-2 receptors). Figure 5 illustrates subsequent events. Four patients responded inadequately to treatment and underwent cardiac transplantation (1 died). After 1 year the remaining 11 had the implanted left ventricular assist device removed. One died within 24 hours (arrhythmia), 1 died 2 years later of lung cancer and 1 received a cardiac transplant 33 months later. The remaining 8 (53% of whole) were followed-up for 4 years; all had normal effort tolerance, were leading normal lives with an EF of 64%.
These remarkable results require confirmation in further studies.
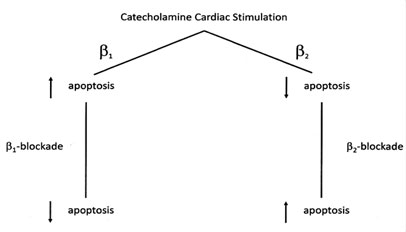 |
| Figure 4 - Effects of beta-1 and beta-2 stimulation and blockade upon myocardial apoptosis/necrosis (55) |
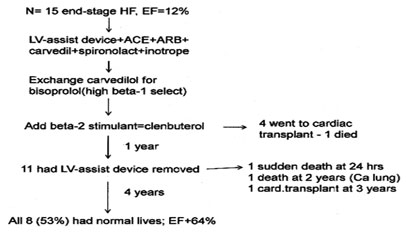 |
Figure 5 - Fifteen end-stage heart failure cases on waiting list for cardiac transplantation ; 8 reponded well to combination of beta-1 blockade (bisoprolol) and beta-2 stimulation (clenbuterol) and were leading normal lives 5 year later (60) |
|
|
Contra-indications to beta-blockers
There are absolute and relative contra-indications (61).
a) Absolute contraindications
i) Advanced heart block, unless a permanent pacemaker is present.
ii) Sinus bradycardia of less than 50 bpm, unless a pacemaker is present.
iii) Systolic blood pressure of less than 85 mm Hg.
iv) Asthma or reversible airways obstruction (though use of a highly beta-1 selective agent, at low-dose, like bisoprolol may be tried (in a hospital environment).
b) Relative contraindications
i) Chronic obstructive pulmonary disease (COPD) is usually not a problem even for non-selective beta-blocking (62).
ii ) Peripheral vascular disease is usually quoted as a relative contraindication. However in patients with intermittent claudication no difference between bisoprolol and the ACE-inhibitor lisinopril could be detected in terms of effects on leg blood flow and vascular resistance and in walking distance (63). Interestingly beta-blockers with vaso-dilator properties, e.g. pindolol and labetalol (additional alpha-blocking properties) were the agents that diminished pain-free walking distance, possibly due to a “vascular steal phenomenon” (64). In terms of prognosis, the UKPDS study (65-6) in obese, diabetic hypertensives the primary end-point of peripheral vascular disease was 48% (trend) more common on captopril compared to the beta-blocker atenolol.
Thus peripheral vascular disease should not be regarded as even a relative contraindication (certainly for beta-1 selective agents), as should diabetes (65-6).
Heart failure primary prevention
As coronary heart disease/myocardial infarction and hypertension are the two main contributors to the appearance of HF, is there evidence to indicate that the administration of beta-blockers in these two conditions results in a reduction in the frequency of HF?
a) Myocardial infarction
i) Early intervention (intravenous followed by oral beta-blocker)
In the large COMMIT trial (67) involving 45,852 cases of acute myocardial infarction, patients were randomised to placebo or intravenous, followed by oral metoprolol for one month. There was a 30% excess of cardiogenic shock, mainly on days 0-1, in those randomised to the beta-blocker. Thus beta-blockade should be administered only to those who are haemodynamically stable.
ii) Late intervention (oral beta-blockers a few days post myocardial infarction and continued for several years)
A meta-analysis (68) showed a small, significant excess (5.9% vs 5.4%) of HF in those randomised to beta-blockade. There is thus no evidence from, randomised studies that beta-blockers prevent HF after late intervention
Table 4: Admission for heart-failure in relation to beta-blocker dose in 8232 elderly post-myocardial infarction patients with no history of heart failure (69) |
|||||||||||||||||||||||||
|
b) Hypertension
i) First-line beta-blockade in younger-middle –aged diastolic hypertensives
Heart failure was not usually a pre-specified primary or secondary end-point, except in the UKPDS study (65-6) involving overweight (mean BMI=30) hypertensives with type-2 diabetes. In UKPDS 38 (65) tight-control of blood pressure (first-line atenolol or captopril) was compared with less tight control; the difference was 10/5 mm Hg. Table 5 shows the effect of tight control, atenolol and captopril vs less tight control of blood pressure upon the frequency of heart failure. The prime contributor to the significant 56% reduction in heart failure was from the beta-blocker (66)
Figure 5 - Fifteen end-stage heart failure cases on waiting list for cardiac transplantation ; 8 reponded well to combination of beta-1 blockade (bisoprolol) and beta-2 stimulation (clenbuterol) and were leading normal lives 5 year later (60) |
||||||||||||
|
|
ii) Second-line beta-blocker (first-line diuretic) in elderly systolic hypertensives
In the SHEP placebo-controlled study (70) in 4,736 elderly patients with isolated systolic hypertension, diuretic first-line/atenolol second-line was associated with a 49% (p<0.001) reduction in the frequency of heart failure. In patients with a previous myocardial infarction the reduction was 80%.
In the vast ALLHAT study involving 33,357 elderly hypertensives (71) diuretic-based (beta-blocker second-line) therapy was significantly superior to both calcium antagonist and ACE-inhibitor-based therapy in preventing heart failure. A follow-up analysis of the ALLHAT study (72) showed that the diuretic/beta-blocker-based therapy was significantly superior to ACE-inhibitor, calcium antagonist and alpha-blocker-based therapy in reducing the frequency of diastolic HF and significantly superior to calcium antagonist and alpha-blocker-based therapy in reducing the frequency of systolic HF – Table 6.
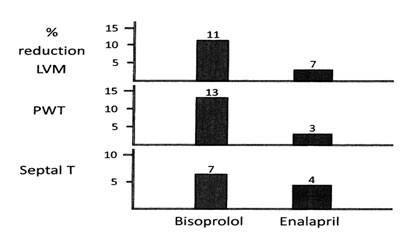
| Figure 6 = Effect of bisoprolol and enalapril, dosed for 6 months, upon LVH in 56 middle-aged hypertensives (93) Figure 7 = Hypertensive response resulting from interaction between epinephrine and non-selective and modestly beta-1 selective beta-blockers (104) |
||||||||||||||||||
|
||||||||||||||||||
iii) Left ventricular hypertrophy (LVH), the remodelling process and heart failure; implications for role of beta-blockers.
LVH is a powerful predictor of HF (73). However not all forms of LVH are dangerous. Appropriate LVH, with normal, wall-stress, it typically associated with athletes and is compatible with a normal life-span (74). Inadequate LVH has a high wall-stress, linked to high blood pressure, and is associated with a poor prognosis in terms of cardiovascular events (75). Inappropriate LVH has a low wall-stress and is not associated with high blood pressure but is closely linked to neurohumoral activation (74) and has a poor prognosis, particularly for HF. Thus inappropriate LVH is associated with very high levels of sympathetic nerve activity (76), particularly in the heart (77-8). Such hearts, under the influence of chronic beta-1 stimulation, go on to develop apoptosis, necrosis and inflammation, fibrosis and myofibrillar-linkage break-down, resulting in a remodelled left ventricle with dilated systolic HF (79). This harmful remodelling process can be prevented by beta-blockade (80). Interestingly angiotensin II-induced cardiac fibrosis acts via norepineprine release and can be modified by beta-1 blockade as well as ACE-inhibition (81).
LVH in elderly isolated systolic hypertension, resulting from decreased arterial compliance (82), is associated with a high central augmented systolic pressure (83). Such LVH is usually concentric and leads on to an inelastic stiff left ventricle, shortness of breath and diastolic HF.
Beta-blockers have a poor image as agents able to reverse LVH. Meta-analysis has indicated the beta-blockers reverse LVH only modestly compared to ACE-inhibitors (84). However such analyses took no account of age. For example atenolol has a total absence of efficacy in reversing LVH (almost certainly concentric) in elderly patients with isolated systolic hypertension(85) (but does when given second-line to first-line diuretic as in the SHEP study (86)). In contrast atenolol is highly effective in reversing LVH (almost certainly eccentric LVH) in younger/middle-aged hypertensives whether assessed by ECG (87) or echocardiography (88), as is metoprolol (89). Bisoprolol was highly effective in reducing left ventricular mass in young/middle aged hypertensives (90-1) with improvement of diastolic function and coronary flow-reserve (92). Bisoprolol was at least as effective as enalapril in reversing echocardiographic LVH (93) – Figure 6.
 |
Choice of beta-blocker for the treatment of heart failure
a) Efficacy
Clearly beta-blockers containing ISA (xamoterol, bucindolol and nebivolol) would be inappropriate as they have failed to significantly reduce all-cause mortality in HF patients (30-2). The three beta-blockers without ISA that have achieved a significant 34-5% decrease in all-cause mortality are bisoprolol (27), metoprolol (28) and carvedilol (29). First-line bisoprolol has also proved to be at least as good as first-line ACE-inhibitor enalapril in reducing all cause death (51) and superior in reducing sudden death (52). There is thus now a genuine choice of first-line agent, ACE-inhibitor or beta-1 blockade, for the treatment of HF. Clearly beta-1 blockade is the active ingredient as regards beta-blocker efficacy in HF. The additional properties of beta-2 blockade (metoprolol and carvedilol) and alpha-blockade (carvedilol) have not been shown to be of benefit in treating HF. All 3 successful beta-blockers effectively antagonise the cardiac beta-1 receptors and the choice would come down to personal preference.
|
b) Adverse Reactions
Most beta-blocker-related adverse reactions are associated with beta-2 blockade and alpha-blockade (e.g. labetalol and carvedilol). It is thus not surprising that marked differences in adverse-reaction profiles exist amongst the range of beta-blockers (94).
i) Metabolic disturbance
Metabolic disturbances involving blood sugar, insulin-resistance, plasma triglycerides, VLDL and HDL result from beta-2 blockade (53, 94). Thus non-selective agents like propranolol will be the worst offenders followed by modestly beta-1 selective agents like metoprolol and atenolol. High beta-1 selectivity (e.g. bisoprolol) or the possession of alpha-blocking properties (e.g. carvedilol) or beta 2/3 ISA (e.g. nebivolol) are associated with minimal or absent metabolic disturbance (53).
ii) Bronchospasm
Beta-2 receptors are present in bronchial muscle and stimulation results in bronchodilatation. The beta-blockers that occupy the bronchial beta-2 receptors will not only increase the risk of bronchoconstriction but will also inhibit the benefits of beta-2 stimulation-induced bronchodilation (94). Modestly beta-1 selective atenolol can induce bronchospasm, this being one of the main causes of drop-outs in the UKPDS study (95). High beta-1 selectivity e.g. bisoprolol at low-dose, is the safest option (96).
iii Weight gain
Increase in weight of 1-2 kg has been noted with beta-blockers and may be linked to a reduction in thermogenesis (94).
iv) Fatigue
Some patients do experience fatigue, particularly with non-selective or partially beta-1 selective agents (94). This will be partly due to the decrease in cardiac output (beta-1 blockade) but also via direct beta-2 blockade within muscle (particularly involving slow-twitch aerobic fibres) which affects lactic acid dynamics (97-8)
v) Smoking interaction
Cigarette-smoking totally abolishes the benefits of non-selective and modestly beta-1 selective beta-blockers in the treatment of hypertension (53). The significant reductions in the frequency of myocardial infarction and cardiovascular end-points by propranolol (99), oxprenolol (100), metoprolol (101) and atenolol (102) were totally negated by smoking.
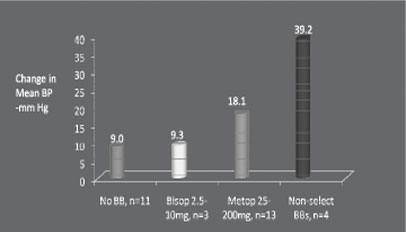 |
Figure 7 = Hypertensive response resulting from interaction between epinephrine and non-selective and modestly beta-1 selective beta-blockers (104) |
vi) Insulin-induced hypoglycaemia/hypertensive interaction
Not only is insulin-induced hypoglycaemia prolonged by non-selective agents (105) but hypertensive responses may also be evoked. As with cigarette smoking, insulin-induced hypoglycaemia is accompanied by an out- pouring of epinephrine (106) which, in the presence of beta-1/2 blockade, can induce hypertensive responses accompanied by marked reflex bradycardia. The hypertensive responses are sometimes marked (approaching 200mm Hg) and may be linked to encephalopathy (107).
vii) Postural hypotension
This is a problem for combined beta-blocker/alpha-blocker agents. Labetalol, with a marked degree of alpha-blocking activity, is the worst offender (94). However dizziness is the most common side-effect with carvedilol in the treatment of heart failure (45). With the elderly hypertensive 25% were unable to stand after the first-dose of carvedilol (108).
|
viii) Impotence and sexual dysfunction
This is certainly the most emotive of potential beta-blocker-induced adverse reactions. There is a powerful placebo-effect to be taken into account (109). Table 7 illustrates the frequency of sexual dysfunction vs placebo with various beta-blockers. Combined beta-1/2/alpha blocking molecules are the most likely to cause sexual problems. In middle-aged men carvedilol induced erectile-dysfunction in 13.5% of cases vs placebo (110). Non-selective propranolol was associated with a 5% withdrawal rate vs placebo in middle-aged hypertensives (99). Moderately beta-1 selective atenolol caused erectile-dysfunction in 3% of subjects vs placebo (109). In middle-aged men sexual dysfunction occurred at placebo-level with highly beta-1 selective bisoprolol (111).
ix) Central nervous system side-effects
Lipophilic beta-blockers like propranolol and metoprolol are detected at high concentration in human brain tissue (112) and are associated with an increased frequency of side-effects such as insomnia, dreams and nightmares (113-4).
| Table7: Beta-blockers and sexual dysfunction vs placebo | |||||||||||||||
|
ix) Central nervous system side-effects
Lipophilic beta-blockers like propranolol and metoprolol are detected at high concentration in human brain tissue (112) and are associated with an increased frequency of side-effects such as insomnia, dreams and nightmares (113-4).
Summary and Conclusions
1. The life-time risk of developing HF is about 20% (40% if hypertension present). With increasing longevity in the developed world the burden of HF (hospitalisation) is set to increase over the next 10-20 years.
2. Coronary heart disease and hypertension are the two main causes of HF; coronary heart disease (and obesity) in the case of systolic HF and hypertension in the case of diastolic HF (mainly in the elderly).
3. Beta-blockers have become the corner-stone (alongside ACE-inhibitors) in the treatment of systolic HF. Bisoprolol, metoprolol and carvedilol (on an ACE-inhibitor background) have reduced all-cause death by 34-5%. The presence of intrinsic sympathomemetic activity (xamoterol, bucindolol, nebivolol) diminishes efficacy in the treatment of systolic HF.
4. First-line bisoprolol has proved “non-inferior” to first-line enalapril in reducing all-cause death and is probably superior in reducing sudden death.
5. The main mode of action of beta-blockers in treating systolic HF is inhibition of chronic beta-1 stimulation-induced myocardial apoptosis/necrosis/inflammation. The combination of pure beta-1 blockade
(low-dose bisoprolol) and pure beta-2 blockade (clenbuterol) may prove invaluable in the treatment of end-stage systolic HF (thus avoiding cardiac transplantation).
6. The appropriate treatment of diastolic HF has yet to be determined.
7. Beta-blockade is effective in the prevention of HF i) in the post-myocardial infarction period and ii) as first-line agents in the treatment of young/middle-aged hypertension and as second-line agents (to first-line diuretics) in the treatment of elderly systolic hypertension. Beta-blockers are highly effective in reversing LVH in young/middle-aged hypertensives (LVH pre-disposes to HF in young/middle-aged hypertension) and are (bisoprolol) at least as good as ACE-inhibitors.
8. Choice of beta-blocker is important as benefit is not a class-effect. Intrinsic sympathomimetic activity (ISA) (xamoterol, bucindolol, nebivolol) markedly diminishes efficacy. The choice is between bisoprolol, metoprolol succinate and carvedilol for optimal efficacy.
9. Adverse reactions are associated, mainly, with beta-2 blockade and alpha-blockade. Thus non-selective (e.g. propranolol) or modestly beta-1 selective (e.g. metoprolol, atenolol) are associated with metabolic disturbance, bronchospasm, epinephrine/hypertensive interaction (with cigarette-smoking or insulin-induced hypoglycaemia), while the possession of alpha-blocking activity (e.g. carvedilol) is associated with dizziness and postural hypotension. The possession of beta-2 blockade, particularly if combined with alpha-blockade, is associated with an increased occurrence of sexual dysfunction. Lipophilic beta-blockers like propranolol and metoprolol appear in high concentrations in human brain tissue and are associated with side-effects such as insomnia, dreams and nightmares.
References
1. McMurray JJ, Pfeffer MA. Heart failure. Lancet 2005;365:1877-89
2. Wood DA. Preventing clinical heart failure: the rationale and scientific evidence. Heart 2002;88(2):15-22.
3. Lloyd-Jones DM, Larson MG, Leep EP et al. Lifetime risk of developing congestive heart failure. The Framingham Heart Study. Circulation 2002;106:3068-72.
4. Levy D, Kenchaiah S, Larson MG et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med 2002;347:1397-402.
4(a). Jhund PS, MacIntyre K, Simpson CR et al Long-term trends in first hospitalisation for heart failure and subsequent survival between 1996 and 2003. Circulation 2009;119:515-23.
5. Stewart S, MacIntyre K, Capewell S, Mc Murray JJ. Heart failure and the aging population an increasing burden in the 21st century? Heart 2003;89:49-53.
6. Gheorghiade M, Sopko G, De Luca L et al. Navigating the crossroads of coronary artery disease and heart failure. Circulation 2006;114:1202-13.
7. Jessup M, Brozena S. Heart failure. N Engl J Med 2003;348:2007-18.
8. Kenchaiah S, Evans JC, Levy D et al. Obesity and the risk of heart failure. N Engl J Med 2002;347:305-13.
9. Stork S, Boivin V, Horf R et al. Stimulating autoantibodies directed against the cardiac beta-1 adrenergic receptor predict increased mortality in idiopathic cardiomyopathy. Am Heart J 2006;152:697-704.
10 Jane-wit D, Altuntas CZ, Johson JM et al. Beta-1 adrenergic receptor autoantibodies mediate dilated cardiomyopathy by agonistically inducing cardiomyocyte apoptosis. Circulation 2007;116:399-410.
11. Jahns R, Boivin V, Siegmund C, Inselmann G, Lohse MJ, Boege F. Autoantibodies activating human beta-1 adrenergic receptors are associated with reduced cardial function in chronic heart failure. Circulation 1999;99:649-54.
12. Blackledge HM, Tomlinson J, Squire IB. Prognosis for patients newly admitted to hospital with heart failure: survival trends in 12,220 index admissions in Leicestershire 1993-2001. Heart 2003;89: 615-620.
|
14. Blackledge HM, Newton J, Squire IB. Prognosis for South Asian and white patients newly admitted to hospital with heart failure in the United Kingdom historical cohort study. BMJ 2003;327:526-30.
15. Wong TJ, Evans JC, Benjamin EJ, Levy D, LeRoy EC, Vasan RS. Natural history of asymptomatic left ventricular systolic dysfunction in the community. Circulation 2003;108:977-82.
15(a). Sanderson JE, Yip GW. Heart failure with a normal ejection fraction. BMJ 2009;338:b52 doi:10. 1136/bmj.b52.
16. Tribouilloy C, Rusinaru D, Mahjoub H et al. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J 2008;29:339-47.
17. Vasan RS. Diastolic heart failure BMJ 2003;327:1181-2.
18. Aurigemma GP, Gaasch WH. Diastolic heart failure. N Engl J med 2004;351:1097-105.
19. Westermann D, Kasner M, Steendijk P et al. Role of left ventricular stiffness in heart failure with normal ejection fraction. Circulation 2008;117:2051-60.
20. Kitzman DW, Little WC, Brubaker PH. Pathophysiological characterization of isolated diastolic heart failure in comparison to systolic heart failure. JAMA 2002;288:2144-50.
21. Haber HL, Simek CL, Gimple LW. Why do patients with congestive heart failure tolerate the initiation of beta-blocker therapy? Circulation 1993;88:1610-19.
22. Greenblatt DJ, Koch-Weser J. Adverse reactions of propranolol in hospitalised medical patients: a report from the Boston Collaborative Drug Surveillance Program. Am Heart J 1973;86:478-84.
23. Ertl G, Neubauer S, Gaudron P et al. Beta-blockers in cardiac failure. Eur Heart J 1994;15 (suppl c):16-24.
24. Chadda K, Goldstein S, Byington R, Curb JD. Effect of propranolol after acute myocardial infarction in patients with congestive heart failure. Circulation 1986;73:503-10.
25. Norweigian Multicentre Study Group. Timolol-induced reduction in mortality and reinfarction in patients surviving acute myocardial infarction. N Engl J Med 1081;304:801-07.
26. Waagstein F, Bristow MR, Swedberg K et al. Beneficial effects of metoprolol in idiopathic dilated cardiomyopathy. Metolpolol in Dilated Cardiomyopathy (MDC) Trial Study Group. Lancet 1993;342:1441-6.
27. CIBIS-II investigators and committee. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet 1999;353:9-13.
28. The MERIT-HR Investigators. Effect of metoprolol CR/XL in chronic heart failure. Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HR). Lancet 1999;35:2001-
29. Packer M, Coats AJ, Fowler MB et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med 2001;344:1651-8.
30. Xamoterol in Severe Heart Failure Study Group. Xamoterol in severe heart failure. Lancet 1990;336:1-6.
31. The Beta-blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N Engl J Med 2001;344:1659-67.
32. Flather MD, Shibata MC, Coats AJ et al. Randomised trial to determine the effect of nebivolol on mortality and cardiovascular hospital admissions in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215-25.
33. Deedwania PC, Gottlieb S, Ghali JK, Waagstein F, Wikstrand JC; for the MERIT-HF Study Group. Efficacy, safety and tolerability of beta-adrenergic blockade with metoptolol CR/XL in elderly patients with heart failure. Eur Herat J 2004;25:1300-09.
34. Goldstien S, Dedwania P, Gottlieb S, Wikstrand J, for the MERIT-HF Study Group. Metoprolol CR/XL in black patients with heart failure (from the metoprolol CR/XL randomised Intervention Trial in Chronic Heart Failure). Am J Cardio 2003;92:478-80.
35. Cruickshank JM, Prichard BNC. Beta-blockers in clinical practice. 2nd edit. Edinburgh Churchill Livingsone, 1994 p 1106-9.
36. Andreka P, Aiyar N, Olson LC et al. Bucindolol displays intrinsic sympathomemetic in Congestive Heart Failure (MERIT-HF). Lancet 1999;353:2001-7.
29. Packer M, Coats AJ, Fowler MB et al. Effect of carvedilol on survival in severe chronic heart failure. New Engl J Med 2001;244:1651-8.
30 Xamoterol in Severe heart Failure. Lancet 1990:336:1-6.
31. The Beta-blocker Evaluation of Survival Trial Investigators. A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. New Engl J Med 2001;344:1659-67.
32. Flather MD, Shibata MC, Coats AJ et al. Randomised trial to determine the effect of nebivolol on mortality and cardiovascular hospital admissions in elderly patients with heart failure (SENIORS). Eur Heart J 2005;26:215-25.
33. Deedwania PC, Gottlieb S, Ghali JK, Waagstein F, Wikstrand JC; for the MERIT-HF Study Group. Efficacy, safety and tolerability of beta-adrenergic blockade with metoprolol CR/XL in elderly patients with heart failure. Eur heart J 2004;25:1300-09.
34. Goldstein S, Deedwania P, Gottlieb S, Wikstrand J, for the MERIT-HF Study Gropup. Metoprolol CR/XL in black patients with heart failure (from the metoprolol CR/XL Randomised Intervention Trial in Chronic heart Failure). Am J Cardiol 2003;92:478-80.
34a Fauchier L, Grimard C, Pierre B et al. Comparison of beta-blocker and digoxin alone and in combination for management of patients with atrial fibrillation and heart failure. Am J Cardiol 2009;103:248-54.
35. Cruickshank JM, Prichard BNC. Beta-blockers in clinical practice. 2nd edit. Edinburgh Churchill Livingstone; 1994 p 1106-9.
36. Andreka P, Aiyar N, Olson LC et al. Bucindolol displays intrinsic sympathiomemetic activity in human muocardium. Circulation 2002;105:2429-34.
37. Maack C, Bohm M, Vlaskin L et al. Partial agonist activity of bucindolol is dependent on the activation state of the human beta-1 adrenergic receptor. Circulation 2003;108:348-53.
38. Brostow MR, Krause-Steinrauf H, Nuzzo R et al. Effect of baseline or changes in adrenergic activity on clinical outcomes on the Beta-blocker Evaluation of Survival Trial. Circulation 2004;110:1437-42.
39. Floras JS. The “unsympathetic” nervous system of heart failure. Circulation 2002;105:1753-5.
40. Dessy C, Moniotte S, Ghisdal P, Havaux X, Noirhomme P, Balligand JL. Endothelial beta-3 adreoceptors mediate vaso relaxation of human coronary microarteries through nitric oxide and endothelium-dependent hyperpolarisation. Circulation 2004;110:948-54.
41. Maffei A, Di Pardo A, Carangi R et al. Nebivolol induces nitric oxide release in the heart through inducible nitric oxide synthase activation. Hypertension 2007;50:652-6.
42. Moniotte S, Kobzik L, Feron O, Trochu J-P, Gauthier C, Balligrad J-L . Upregulation of beta-3 adrenoceptors and altered contractile response to inotropic amines in human failing myocardium. Circulation 2001;103:1649-55.
43. Gauthier C, Leblais V, Kobzik L et al. The negative inotropic effect of beta-3 adrenoceptor stimulation is mediated by actvation of a nitric oxide synthase pathway in human ventricle. J Clin Invest 1998;102:1377-84.
44. Schulman SP, Becker LC, Kass DA. L-Arginine therapy in acute myocardial infarction. JAMA 2006;295:58-64.
45. Packer M, Bristow MR, Cohn JM et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. New Engl J Med 1996;334:1349-55.
46. The CAPRICORN Investigators. Effect of carvediolol on outcome after myocardial infarction in patients with left-ventricular dysfunction the CAPRICORN randomised trial. Lancet 2001;357:1385-90.
47. Shaddy RE, Boucek MM, Hsu DT at al. Carvedilol for children and adolescents with heart failure. JAMA 2007;298:1171-79.
48. Poole-wilson PA, Swedberg K, Cleland JG et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the carvedilol or metoprolol European Trial (COMET): randomised controlled trial. Lancet 2003;362:7-13.
49. Dargie HJ. Beta-blockers in heart failure Lancet 2003;362:2-3.
50. Correspondence. COMET: a proposed mechanism of action to explain the results and concerns about dose. Lancet 2003;362:1076-8.
51. Willenheimer R, van Velduisen JJ, Silke B et al. Effect on survival and hospitalisation of initiating treatment of chronic heart failure with bisoprolol followed by enalapril, as compared with the opposite sequence. Results of the Randomised Cardiac Insufficiency Study (CIBIS III). Circulation 2005;112:2426-35.
52. CIBIS III trial. Bisoprolol treatment for congestive heart failure leads to 46% reduction in sudden death after one year. Cardiovasc. JS Afr 2006;17:278.
53. Cruickshank JM. Are we misunderstanding beta-blockers. Int J Cardio 2007;120:10-27.
54. Bellenger NG, Rajappan K, Rahman SL et al. Effect of carvedilol on left-ventricular remodelling in chronic stable heart failure: a cardiovascular magnetic resonace study. Heart 2004:90:760-4.
55. Communal C, Singh K, Sawyer DB, Colucci WS. Opposing effects of beta-1 and beta-2 adrenergic receptors on cardiac myocyte apoptosis. Circulation 1999;100:2210-2.
56. Cruickshank JM, Degaute JP, Kuurne T et al. Reduction in stress/catecholamine-induced cardiac necrosis by beta-1 selective blockade. Lancet 1987;ii:585-9.
57. Reiken S, Gabujakova M, Gaburjakova J et al. Beta-adrenergic receptor blockers restore cardiac calcium release channel (ryanodine receptor) structure and function in heart failure. Circulation 2001;104:2843-8.
58. Gong H, Hong S, Koch WJ et al. Specific beta-2 blocker ICI 118,551 actively decreases contraction through a Gi-coupled form of the beta-2 AR in myocytes from failing human heart. Circulation 2002;105:2497-503.
59. Anyanwu A, Treasure T. Prognosis after heart transplantation. Transplants alone cannot be the solution for end stage heart failure. BMJ 2003;326:509=10.
60. Birks EJ, Tansley PD, Hardy J et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med 2006;355:1873-84.
60(a).Henandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF Registry. J Am Coll Cardiol 2009;53:184-92.
60(b). Voors AA, de Jong M. Treating diastolic heart failure. Heart 2008;94:971-2.
61. Gheorghiade M, Colucci WS, Swedberg K. Beta-blockers in chronic heart failure. Circulation 2003;107:1570-5.
62. Shelton RJ, Rigby AS, Cleland JG, Clark AL. Effect of a community heart failure clinic on uptake of beta-blockers by patients with obstructive airways disease and heart failure. Heart 2006;92:331-6.
63. van de Ven LL, van Leeuwen JT, Smit AJ. The influence of chronic treatment with beta-blockade and angiotensin converting enzyme inhibition on the peripheral blood flow in hypertensive patients with and without concomitant intermittent claudication. J Vasc Dis 1994;23:357-62.
64. Roberts DH, Tsao Y, McLoughlin GA, Breckenridge A. Placebo-controlled comparison of captopril, atenolol, labetalol, and pindolol in hypertension complicated by intermittent claudication. Lancet 1987;2:650-3.
65. UK Prospective Diabet4s Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703-13.
66. UK prospective Diabetes Study Group. Efficacy of atenolol and captopril in reducing risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 39. BMJ 1998, 317:713-20.
67. COMMIT (Clopidogrel and Metoprolol in Myocardial Infarction Trial). Early intravenous and oral metoprolol in 45,852 patients with acute myocardial infarction; randomised placebo-controlled trial. Lancet 2005;366:1622-32.
68. Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta-blockade during and after myocardial infarction: an over view off the randomise trials. Prog Cardiovasc Dis 1985;XXVII (5):335-71.
69. Rochon PA, Tu JV, Anderson GM et al. Rate of heart failure and 1-year survival for older people receiving low-dose beta-blocker therapy after myocardial infarction. Lancet 2000;356:639-44.
70. Kostis JB, Davis BR, Cutler J et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA 1997;278:212-6.
|
72. Davis BR, Kostis JM, Simpson LM et al: ALLHAT Collaborative Research Group. Heart Failure with preserved and reduced left-ventricular ejection fraction in the antihypertensive and lipid-lowering treatment to prevent heart attack trial. Circulation 2008;118:2259-67.
73. Anderson K, Wilson PW, Odell PM, Kannel WB. An updated coronary risk profile-a statement for Health Professions. Circulation 1991;83:356-62.
74. Muiesan MI, Salvetti M, Paini A et al. Inappropriate left-ventricular mass changes during treatment adversely affects cardiovascular prognosis in hypertensive patients. Hypertension 2007;49:1077-83.
75. Sugishita Y, Aida K, Ohtsukas, Yamaguchi I. Ventricular wall stress revisited. A keystone of cardiology. Jpn Heart J 1994;35:577-87.
76. Burns J, Sivananthan MV, Ball SG, Mackintosh AF, Marg DA, Greenwood JP. Relationship between central sympathetic drive and MRI imaging-determined left-ventricular mass in essential hypertension. Circulation 2007;115:1999-2005.
77. Kelm M, Schafer S, Mingers S et al. Left -ventricular mass is linked to cardiac noradrenaline in normotensive and hypertensive patients. J Hypertens 1996;14:1365-7.
78. Schlaich MP, Kaye DM, Lambert E, Somerville M, Socratous F, Esler MD. Relation between cardiac sympathetic activity and hypertensive left-ventricular hypertrophy. Circulation 2003;108:560-5.
79. Seeland V, Selejan S, Engelhardt S, Muller P, Lohse MJ, Bohn M. Interstitial remodelling in beta-1 adrenergic receptor trans-genic mice. Basic Res Cardio 2007;102:183-93.
80. Asai K, Yang EP, Geeng YJ et al. Beta adrenergic blockade arrests myocardial damage and preserves cardiac function in the trans-genic G (salpha) mouse. J Clin Invest 1999;104:551-8.
81. Tallaj J, Wei C-C, Hankes GH et al. Beta-1 adrenergic receptor blockade attenuates angiotensin II-mediated catecholamine reddlease into the cardiac interstitium in mitral regurgitation. Circulation 2003;1089:225-30.
82. Franklin SS, Pio JR, Wong ND, Larson MG, Leip EP, Vasan RS. Predictors of new-onset diastolic and systolic hypertension. The Framingham heart Study. Circulation 2005;111:1121-7.
83. Agabiti-Rosei E, Marcia G, O’Rourke MF et al. Central blood pressure measurements and antihypertensive therapy. A consensus document. Hypertens 2007;50:154-60.
84. Cruickshank JM, Lewis J, Moore V, Dodd C. Reversibility of left-ventricular hypertrophy by differing types of antihypertensive therapy. J Hum Hypertens 1992;6:85-90.
85. Schulman SP, Weiss JL, Becker LC et al. Effect of antihypertensive therapy on left-ventricular mass in elderly patients. N Engl J Med 1990;322:1350-6.
86. Offili EO, Cohen JP, Vrain JA et al. Effect of treatment of isolated systolic hypertension on left-ventricular mass. JAMA 1998;279:778-80.
87. Cruickshank JM, Higgins TJ, Pennart K, Thorpe JM, Zacharias FM, Zacharias FJ. The efficacy and tolerability of antihypertensive treatment based on atenolol in the prevention of stroke and the regression of left-ventricular hypertrophy. J Hum Hypertens 1987:1:87-93.
88. Otterstad JE, Froeland G, Soeyland AK, Kautsen KM, Ekeli T. Changes in left-ventricular dimensions and systolic function in 100 mildly hypertensive men during one year’s treatment with atenolol vs hydrochlorthiazide and amiloride. J Intern Med 1992;231:493-501.
89. Franz IW, Behr V, Ketelhut R, Tonnesmann V. Decreasing the antihypertensive dosage during long-term treatment and complete regression of left-ventricular hypertrophy. Dtsch Med Wochenschr 1996;121:472-7.
90. de Teresa E, Gonzalez M, Camacho-Vasquez C, Tabuenca MJ. Effects of bisoprolol on left-ventricular hypertrophy in essential hypertension. Cardivasc Drugs Ther 1994;8:837-43.
92. Motz W, Vogt M, Scheler S, Schwartzkopff B, Strauer BE. Improvement in coronary reserve following regression of hypertrophy resulting from blood pressure lowering with a beta-blocker. Dtsch Med Wochen. 1993;118:535-40.
93. Goss P, Routaut R, Herreo G, Dallochio M. Beta-blockers vs ACE-inhibition; effects on left-ventricular hypertrophy. J Cardiovasc Pharmacol 1990;16(S); S145-50.
94. Cruickshank JM, Prichard BN. Beta-blockers in clinical practice. 2nd edition. Edinburgh, Churchill Livingsone;1994:907-1053.
95. UK Prospective Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. BMJ 1998;317:703-13.
96. Dorrow P, Bethge M, Tonnesmann V. Effects of single doses of bisoprolol and atenolol on airways function in non-asthmatic chronic obstructive lung disease and angina pectoris. Eur J. Clin Pharmacol 1983;31:143-7.
97. Clausen T, Flatman JA. Beta-2 adrenoceptors mediate the stimulating effect of adrenaline on active electrogenic Na-K transport in rat soleus muscle. Br J Pharmacol 1980;68:749-55.
98. Frisk-Holmberg M, Jorfeldt L, Juhlin-Dannfelt A. Metabolic effets in muscle during antihypertensive therapy with beta-1 and beta-1/-2 adrenoceptor blockers. Clin Pharmacol Ther 1981;30:611-18.
99. Medical Research Council Working Party. MRC trial of treatment of mild hypertension: principal results, BMJ 1985;291:97-104.
100. IPPPSH Collaborative Group. Cardiovascular risk and risk factors in a randomised trial of treatment based on the beta-blocker oxprenolol. J Hypertens 1985;3:379-92.
101. Wikstrand J, Warnold T, Tuomilehto J et al. Metopolol versus diuretic in hypertension. Morbidity results from MAPHY study. Hypertension 1991;17:579-88.
102. Medical Research Council Working Party. MRC trial of treatment of hypertension in older adults: principal results. BMJ 1992;304:405-12.
103. Cryer PE, Haymond MW, Satiago JV, Shar SP. Norepinephrine and epinephrine release and adrenergic mediation of smoking associated haemodynamic and metabolic events. N Engl J Med 1976;295:573-7.
104. Tarnow J, Muller RK. Cardiovascular effects of low-dose epinephrine infusions in relation to the extent of pre-operative beta-adrenoceptor blockade. Anaesthesiology 1991;74:1035-43.
105. Deacon SP, Barnett D. Comparison of atenolol and propranolol during insulin-induced hypoglycaemia. BMJ 1976;2:272-3.
106. Lloyd-Mostyn RH, Oram S. Modification by propranolol of cardiovascular effects of induced hypoglycaemia. Lancet 1975;1:1213-5.
107. McMurty RJ. Propranolol, hypoglycaemia and hypotensive crises. Ann Intern Med 1974;80:669-70.
108. Krum H, Conway EL, Broadbear JH, Howes LG, Louis WJ. Postural hypotension in elderly patients given carvedilol. BMJ 1994;309:775-6.
109. Silvestri A, Galetta P, Cerquetari E et al. Report of erectile dysfunction after therapy with beta-blockers is related to patient knowledge of side-effects and is reversed by placebo. Eur Heart J 2003;24:1928-32.
110. Fogari R, Zoppi A, Poletti L, Marasi G, Mugellini A, Corradi L. Sexual activity hypertensive men treated with valsartan or carvedilol: a cross-over study. Am J Hypertens 2001;14:27-31.
111. Broekman CP, Haensel SM, van den Ven LL, Slob AK. Bisoprolol and hypertension: effects on sexual functioning in men. J Sex and Marital Therapy. 1992;18:325-31.
112. Neil-Dwyer G, Bartlett J, McAinsh JH, Cruickshank JM. Beta-adrenergic blockers and the blood-brain barrier. Br J Clin Pharmac. 1981;11:549-53.
113. Kostis JB, Rosen RC. Central nervous system effects of beta-adrenergic blocking drugs; the role of ancillary properties. Circulation 1987;75:204-12.
114. Cruickshank JM, Prichard BN. Beta-blockers in clinical practice. Second edition. Edinburgh, Churchill Livingstone; 1994:940.
|