Recent Landmark Trials
Manish Bansal*, Rahul Mehrotra*, H K Chopra#, Ravi R Kasliwal*Ravinder S Sambi , Krishna n. k.
Medanta- The Medicity, Gurgaon, Haryana.Moolchand Medcity, New Delhi.
ASTRAL (Angioplasty and Stenting for Renal Artery Lesions)
The ASTRAL Investigators. N Engl J Med 2009;361:1953-62
Trial summary:
This trial was conducted to evaluate percutaneous renal artery intervention compared with medical therapy in patients with significant renal artery stenosis.
In this randomized un-blinded trial, 806 patients with significant atherosclerotic renal artery disease were assigned either to undergo percutaneous revascularization in addition to medical therapy (n=403) or remain on medical therapy alone (n=403). Mean follow-up period was 27 months. Renal function, as assessed by the reciprocal of the serum creatinine level (which bears a linear relationship to creatinine clearance) was the primary outcome. Secondary outcomes were blood pressure control, time to first renal event, time to first CV event and mortality.
During the 5 years of follow up, there was no significant difference in the primary outcome between the patients who underwent successful revascularization and those who received medical therapy only. Also, in the various subgroups studied- based on serum creatinine, estimated glomerular filtration rate, kidney length, bilateral renal artery stenosis and stenosis of single functioning kidney, there was no significant difference in the primary outcome between the two groups.
The mean systolic blood pressure was 1.6 mm Hg lower in the revascularization group than in the medical-therapy group (p value=0.06). The mean diastolic blood pressure decreased in both groups, but the reduction was greater in the medical-therapy group. There was no difference in time to first renal event (hazard ratio in the revascularization group- 0.97; p=0.88), time to first CV event (hazard ratio in the revascularization group, 0.94, p=0.61) or in overall survival (103 deaths in revascularization group and 106 deaths in medical therapy group, p=0.46). A total of 38 peri-procedural complications (occurring within 24 hours of the procedure) were reported in the patients undergoing revascularization and nineteen of these were serious (including pulmonary edema, myocardial infarction, renal artery occlusion, peripheral gangrene and amputation).
In conclusion, for patients with atherosclerotic renal artery disease, no significant clinical benefit of revascularization was found up to 5 years of follow up for renal function, reduction of CV events, blood pressure reduction or for mortality. Besides, patients undergoing revascularization were at risk of having serious complications.

Perspective:
Endovascular interventions are associated with significant morbidity, cost and inconvenience to the patient. The benefit of percutaneous intervention in atherosclerotic renovascular disease for improvement of renal function and blood pressure control was claimed on the basis of few non-randomized trials or small randomized studies. In the present study, no worthwhile clinical benefit of renal revascularization could be demonstrated up to five years after intervention in comparison to medical therapy alone. Moreover, renal intervention was found to be associated with substantial risk of serious complications. Therefore, the validity of intervention for renal artery stenosis can now be questioned based on the results of this large randomized trial.
CHAMPION- PLATFORM (Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition- PLATFORM) trial
Bhatt DL, et al. N Engl J Med 2009;361:2330-41
And
Correspondence: Dr Ravi R Kasliwal, chairman, Clinical and preventive Cardiology #9.3rd floor OPD Blcok medanta - the medictity Gurgaon , haryana 122001, India
E-mail: rrKasliwal@hotmail.com
|
CHAMPION- PCI (Cangrelor versus Standard Therapy to Achieve Optimal Management of Platelet Inhibition- PCI) trial
Harrington RA, et al. N Engl J Med 2009;361:2318-29
Platelet inhibition with ADP- receptor blockers is a standard adjuvant therapy in patients undergoing PCI as it significantly improves clinical outcomes in such patients. In addition, these agents also reduce risk of adverse CV events in patients presenting with ACS irrespective of whether PCI is performed. Currently, clopidogrel is the ADP-receptor blocker of choice because of its efficacy and safety profile. However, clopidogrel has several important limitations. It has relatively slow onset of action requiring a loading dose when immediate PCI is planned and produces irreversible inhibition of platelets which becomes a major source of concern if patient has to undergo coronary artery bypass grafting. In addition, recent reports have shown that there is marked variability in its antiplatelet effect in different patients which may be because of genetic polymorphisms, interaction with concomitant drugs, food etc. Therefore, newer antiplatelet agents are being evaluated that may have superior efficacy and better safety profile than clopidogrel. Prasugrel, Ticagrelor and Cangrelor are some of the newer agents that appear promising. The two CHAMPION trials were conducted to evaluated efficacy and safety of cangrelor, an intravenous, rapid-acting, reversible ADP-receptor blocker, in patients undergoing PCI.
CHAMPION-PLATFORM
The CHAMPION-PLATFORM trial compared cangrelor with placebo in patients with non-ST elevation ACS who were undergoing PCI. A total of 5362 patients were randomized to receive either cangrelor infusion or matching placebo at the time of procedure. The patients in the cangrelor arm also received 600 mg clopidogrel at the end of infusion whereas those in the placebo arm received 600 mg clopidogrel at the end of procedure. Primary end-point was a composite of death, MI and urgent revascularization at 48 hours.
Although there was no significant difference between the cangrelor and placebo arms in primary end-point (7.0% vs 8.0%, p=0.17), cangrelor significantly reduced the risk of death (0.2% vs 0.7%, p=0.02) and stent thrombosis (0.2% vs 0.6%, p=0.02). There was no difference in the risk of major bleeding according to GUSTO and TIMI definitions but ACUITY-defined major bleeding episodes increased with cangrelor (5.5% vs 3.5%, p<0.001), mainly because of more groin hematomas.

CHAMPION-PCI
CHAMPION-PCI was a similar trial as CHAMPION-PLATFORM and was conducted in identical patient population with almost similar study design (but also included patients with ST-elevation MI).
In CHAMPION-PCI trial, a total of 8716 patients were randomized to receive either cangrelor infusion or 600 mg clopidogrel at the time of procedure. The patients in the cangrelor arm also received 600 mg clopidogrel at the end of infusion.
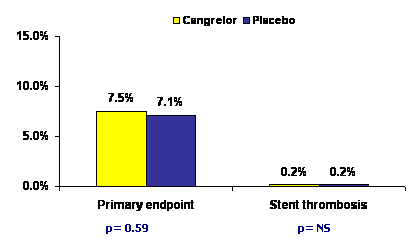
At 48 hours, there was no difference between the two arms in the rate of primary end-point which was a composite of death, MI and urgent revascularization (7.5% with cangrelor vs 7.1% with clopidogrel, p=0.59). Death from any cause and urgent revascularization showed a statistically non-significant trend towards improvement with cangrelor. The risk of major bleeding defined by ACUITY-criteria increased with cangrelor (3.6% vs 2.9%, p=0.06).
Conclusions
Cangrelor is a potent, rapid-acting antiplatelet agent whose action is completely reversed within 60 min of stopping the infusion. These properties make it ideally suited for use in unstable patients undergoing PCI. However, contrary to expectations, the two CHAMPION trials did not find cangrelor superior to clopidogrel in ACS patients undergoing PCI. Several study-design related issues such as timing of clopidogrel loading dose, duration of cangrelor infusion, definition of end-points and strategy for transition from intravenous cangrelor to oral clopidogrel, etc. could have been responsible for the negative results of these trials. Further studies are therefore required to clearly define role of cangrelor in patients undergoing PCI.
COMPARE: Second-Generation Everolimus-Eluting and Paclitaxel-Eluting Stents in Real-Life Practice
Kedhi E, Joesoef KS, McFadden E, et al. Lancet 2010;375:201-9.
Trial summary:
This trial compared the safety and efficacy of second generation everolimus-eluting and paclitaxel-eluting stents in real life situation.
|
In this single centre randomized trial, 1800 consecutive patients undergoing PCI were assigned to treatment with everolimus-eluting stent (EES, n=897) or the paclitaxel-eluting stent (PES, n=903). The primary end point was a composite of all cause mortality, myocardial infarction and target vessel revascularization within 1 year of follow-up.
Primary end point occurred in 6% of the patients in the EES group and in 9% of patients in the PES group (relative risk 0·69, p value =0·02). EES group also had significantly less myocardial infarctions (3% vs 5%, relative risk 0·52, p=0·007), stent thromboses (<1% vs 3%, relative risk 0·26, p=0·002) and target vessel revascularizations (2% vs 6%, relative risk 0·39, p=0·0001) compared to PES group.
Cardiac death, non-fatal myocardial infarctions and target lesion revascularizations were also significantly less in EES group (5%) as compared to PES (8%) group. There was however no difference in the rates of all cause mortality and cardiac mortality.

Perspective:
There had been significant interest since the arrival of EES system with an open cell, thin strut design. This EES was shown to have significantly less incidence of serious cardiac events as compared to first generation PES in a randomized trial published in 2008. There have been improvements in stent design of PES since then and this trial compared the second generation PES and EES in real life situation. The EES system proved to be superior in terms of safety as well as efficacy at 1 year. This difference was a result of reduction in the rate of myocardial infarction, a safety component of the primary endpoint, and reduction in repeat revascularization of the target vessel in this trial.
In conclusion, this trial has demonstrated that everolimus-eluting Xience V stent is better than the second-generation paclitaxel-eluting Taxus Liberté stent in treatment of patients in real-life practice and paclitaxel-eluting stents should no longer be used in everyday clinical practice.
PACE (Pacing to Avoid Cardiac Enlargement)
Yu CM, Chan J, Zhang Q, et al. N Engl J Med 2009; 361:2123-2134.
Trial summary:
This trial was conducted to compare biventricular pacing versus RV pacing in patients with bradycardia due to sinus node dysfunction or advanced atrioventricular node disease with normal ejection fraction.
In this multicenter, randomized, double-blind, prospective study, 177 patients who were successfully implanted with a biventricular pacemaker were assigned to receive atrial synchronized biventricular pacing (89 patients) or RV apical pacing (88 patients). The primary end point was LV ejection fraction and LV end-systolic volume at the end of 12 months. Secondary end points were hospitalization for heart failure, distance walked in 6 minutes and quality of life.
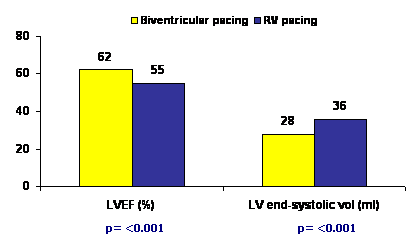
At the end of 12 months, mean LV ejection fraction was significantly lower in the RV pacing group (55%) than in the biventricular pacing group (62%), (p<0.001). There was an absolute reduction in the LV ejection fraction of 6.7% in the RV pacing group from baseline while no change was observed in the biventricular pacing group. The LV end-systolic volume was also significantly more at 12 months in the RV pacing group than in the biventricular pacing group (P<0.001). LV end-systolic volume increased by 7.1 ml (a relative increase of 26%) from baseline to 12 months in the RV pacing group but remained unchanged in the biventricular pacing group. There were no peri-procedural deaths. Hospitalization for heart failure within 12 months occurred in 6% versus 7% patients on biventricular pacing and RV pacing, respectively. Distance in 6-minute walk test was 380 m versus 374 m (p = 0.81) and quality of life was similar between the groups (p = 0.75).
Perspective:
RV apical pacing has been used as a standard mode of ventricular pacing for several decades but its detrimental effect on LV function has only been recognized in the last few years. Abnormal electrical activation results in LV mechanical dyssynchrony, asymmetrical hypertrophy, mitral regurgitation and reduced ejection fraction. This trial clearly demonstrates that the use of RV apical pacing in patients with normal LV systolic function results in deterioration of LV ejection fraction as early as one year after implantation while the use of biventricular pacing preserves LV ejection fraction.
Although, the sample size is small, this trial demonstrates that routine use of biventricular pacing in all patients with bradycardia should be preferred over RV apical pacing to maintain LV ejection fraction and dimensions. However, the complications and cost involved in biventricular pacemaker implantation need to be addressed before this can become a routine strategy in patients with bradycardia.
|
PLATO (Platelet Inhibition and Patient Outcomes)
Wallentin L, et al. N Engl J Med 2009;361:1045-57
Trial summary:
This multi-center, randomized, double-blind study was conducted to compare ticagrelor, a direct inhibitor of platelet receptor P2Y12, with clopidogrel in patients presenting with ACS.
In this study, 18624 patients with ACS (with or without ST-segment elevation) were randomized to receive ticagrelor (180 mg loading dose followed by 90 mg twice daily) or clopidogrel (300 to 600 mg loading dose followed by 75 mg once daily). Patients were flowed for 12 months.

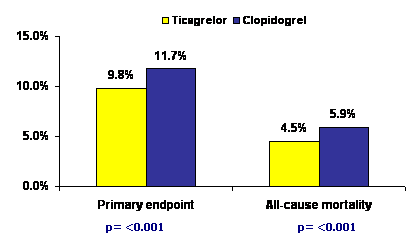
At 12 months, there was significant reduction in the composite primary end-point of CV death, myocardial infarction and stroke with ticagrelor as compared to clopidogrel (9.8% vs 11.7%, p <0.001). The beneficial effect on primary end-point was noted in patients undergoing invasive treatment (8.9% vs 10.6%, p= 0.003). Individual end-points including all-cause mortality (4.5% vs 5.9%, p <0.001), CV mortality (4.0% vs 5.1%, p=0.001) and myocardial infarction (5.8% vs 6.9%, p 0.005) were also decreased significantly with ticagrelor. In patients undergoing coronary stenting, the risk of stent thrombosis was also lower in the ticagrelor group than in the clopidogrel group (1.3% vs 1.9%). No significant difference was found between ticagrelor and clopidogrel in the incidence of major bleeding (11.6% vs 11.2%) but non-coronary bypass surgery related major bleeding increased with ticagrelor (4.5% vs 3.8%, p=0.03).
Perspective:
As already discussed, clopidogrel has been shown to improve clinical outcomes when combined with aspirin in patients presenting with ACS. The benefit is greater in patients undergoing coronary stenting, to whom clopidogrel is prescribed routinely. However, heterogeneity in patient response to clopidogrel has recently emerged as an important factor determining its efficacy. Ticagrelor is another direct-acting inhibitor of P2Y12 receptor and results in faster, greater and more consistent platelet inhibition. The PLATO trial has shown superior efficacy of ticagrelor with a favorable safety profile as compared to clopidogrel in a broad range of patients with ACS. These results make ticagrelor a promising alternative to clopidogrel in such patients. However, patient compliance may be an issue with ticagrelor as it requires twice- daily dosing whereas clopidogrel needs to be given once-daily only.
RE-COVER (Efficacy and Safety of Dabigatran Compared to Warfarin for 6-Month Treatment of Acute Symptomatic Venous Thromboembolism)
Schulman S, et al. N Engl J Med 2009;361:2342-52
Trial summary:
This primary purpose of RECOVER trial was to determine safety and efficacy of orally active direct thrombin inhibitor dabigatran with warfarin in treatment of acute VTE.
A total of 2,539 patients with acute VTE were randomized to dabigatran 150 mg twice a day (n=1274) or warfarin (n=1265) after initial therapy with intravenous un-fractionated heparin or subcutaneous low molecular weight heparin. Warfarin dose was adjusted to achieve an INR of 2.0 to 3.0. The patients were followed up for 6 months. The primary outcome was the 6-month incidence of recurrent symptomatic, objectively confirmed venous thromboembolism or death due to recurrent VTE.
At six months, the primary outcome of recurrent VTE or death due to recurrent VTE occurred in 2.4% of patients in the dabigatran arm and 2.1% of patients in the warfarin arm (hazard ratio 1.1, p < 0.001 for noninferiority). There was no difference between the two groups in all-cause mortality (1.6% vs. 1.7%, p > 0.05) major bleeding episodes (1.6% vs. 1.9%, p = 0.38), acute coronary syndromes or derangement in liver-function tests. However, incidence of any bleeding was lower in the dabigatran arm (5.6% vs. 8.8%, p = 0.002). Adverse events leading to discontinuation of the study drug was commoner with dabigatran (9.0%) as compared to warfarin (6.8%, p = 0.05). On an average, 16 INR checks were required in the warfarin group during the 6-months period.
|
Perspective:
Oral anticoagulation with warfarin is an integral component of therapy in a number of clinical settings- patients with prosthetic heart valves, atrial fibrillation, acute and chronic VTE, etc. The major problem with use of warfarin is the requirement of regular INR monitoring to ensure optimum anticoagulation without significantly increasing the risk of bleeding complications. Oral dabigatran, which does not have any significant interaction with food or other drugs, offers a major advantage over warfarin as it does not require regular dose-monitoring. In a recent trial, oral dabigatran was shown to be superior to warfarin in prevention of stroke in patients with atrial fibrillation. The present trial has provided another evidence to support its efficacy and safety as an oral anticoagulant. In view of these results, coupled with its ease of use, dabigatran is likely to find increasing role in clinical practice very soon.
Efficacy and safety of Varenicline for smoking cessation in patients with coronary artery disease.
Rigotti NA, Pipe AL, Benowitz NL, Arteaga C, Garza D, Tonstad S. Circulation 2010;121:221-9.
Trial summary:
This trial was conducted to evaluate safety and efficacy of varenicline, a partial α4β2 nicotinic acetylcholine receptor agonist helpful in achieving smoking cessation, when compared with placebo in patients with stable CAD.
In this multicenter, randomized, double-blind, placebo-controlled trial, 714 patients (35-75 years) with stable CAD who had smoked ≥ 10 cigarettes daily in the previous one year and wanted to quit smoking were enrolled. All patients were given counseling for smoking cessation and were randomized to receive varenicline 1 mg twice daily (n=355) or placebo (n=359) for a period of 12 weeks. The follow up was up to 52 weeks and primary end point was carbon monoxide confirmed continuous abstinence rate in the last 4 weeks (week 9-12) of treatment.

The continuous abstinence rate was significantly higher for varenicline than placebo during weeks 9 through 12 (47.0% versus 13.9%; p=<0.0001). Although abstinence reduced with time, it remained better with varenicline as compared to placebo (28.2% versus 9.5% (p < 0.001) at weeks 9-24 and, 19.2% versus 7.2% (p < 0.001) at weeks 9-52. There was no significant difference in the varenicline and placebo groups in CV mortality (0.3% versus 0.6%), all-cause mortality (0.6% versus 1.4%), CV events (7.1% versus 5.7%), or serious adverse events (6.5% and 6.0%). The study medication was stopped by 9.6% of patients in the varenicline group and 4.3% in the placebo group. The incidence of common adverse events- nausea (29.5% vs. 8.6%), vomiting (8.2% vs. 1.1%) and insomnia (11.9% vs. 6.6%), was higher with varenicline as compared to placebo.
Thus, this trial shows that varenicline is safe and effective pharmacotherapy for achieving smoking cessation in patients with stable CAD who wish to quit smoking.

Perspective:
Cigarette smoking is a major risk factor for CAD and is the key component of primary and secondary CVD prevention programs. While smoking cessation is associated with significant reduction in risk of death (36% in patients with CAD), it has been difficult to achieve and maintain abstinence in smokers. Varenicline, a partial agonist of acetylcholine receptors, has been shown to be effective and superior to other therapies for smoking cessation like bupropion and nicotine replacement therapy but this has been shown only in healthy smokers and the safety of varenicline had never been demonstrated in patients with CVD. This trial reinforces the efficacy of varenicline and also documents its safety in patients with stable CAD.
|