Landmark Trails.
Manish Bansal, Ravi R. Kaliwal, H.K. Chopra, R.S. Sambi, Krishna C.K.
Indraprastha Apollo Hospital,New Delhi
Moolchand Medcity, New Delhi.
ECASSIII (European Cooperative Acute Stroke Study III)
Hacke W, et al. N Engl J Med 2008;359:1317-29
Trial summary:
This trial was conducted to evaluate role of thrombolysis in patients with acute ischemic stroke presenting beyond the currently accepted window period of 3 hours.
Over 800 patients presenting with non-severe ischemic stroke between 3 and 4.5 hours of symptom onset were randomized to intravenous alteplase (0.9 mg/kg, n=418) or placebo (n=403). Patients were followed for 3 months. Disability as assessed using modified Rankin score (0-1 versus 2-6) was the primary outcome.
At 3 months, the primary outcome (0-1 on modified Rankin score) was better with alteplase as compared to placebo (52.4% vs. 45.2%, OR 1.34, 95% CI 1.02-1.76, p = 0.04). The risk of intracranial hemorrhage was also higher with alteplase vs. placebo (for any intracranial hemorrhage, 27.0% vs. 17.6%; p=0.001; for symptomatic intracranial hemorrhage, 2.4% vs. 0.2%; p=0.008). However, there was no difference in the mortality in the two groups. It was estimated that for 1 patient to have a favorable outcome (a score of 0 or 1 on the modified Rankin scale), the number needed to treat was 14 with the extended time window.
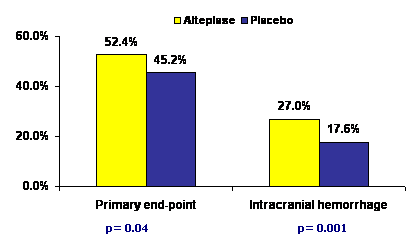
In conclusion, alteplase was associated with better neurological outcomes than placebo in patients with non-severe ischemic stroke presenting between 3 and 4.5 hours of symptom onset but the risk of intracranial bleeding was higher.
Perspective:
Currently, thrombolysis in patients with ischemic stroke is recommended only in those presenting within first 3 hours of symptom-onset. However, significant proportion of patients present late after symptom onset and are therefore not thrombolysed. In the present study, thrombolysis with alteplase between 3 to 4.5 hours of symptom-onset in patients with non-severe stroke produced clinically meaningful overall benefit. This finding advocates extension of the treatment window for patients who do not arrive at the hospital early. However, it must be noted that every effort should be made to keep the "door-to-needle" time as short as possible so as to derive the maximum benefit.
HORIZONS-AMI
(Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction study)
Stone GW, et al. N Engl J Med 2008;358:2218-30
Trial summary:
This trial was conducted to evaluate safety and efficacy of bivalirudin (a direct thrombin inhibitor) instead of a combination of heparin and glycoprotein IIb-IIIa inhibitors in patients with STEMI undergoing primary PCI.
In this open-label study, 3602 patients with STEMI undergoing primary PCI were randomized to bivalirudin (n = 1,800) or heparin plus a glycoprotein IIb-IIIa inhibitor (n = 1,802). The two primary end points of the study were major bleeding and net adverse clinical events defined as a composite of major bleeding, death, re-infarction, target-vessel revascularization for ischemia, and stroke within 30 days.
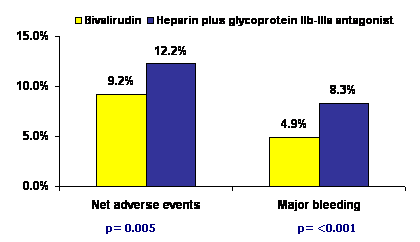
Treatment with bivalirudin, as compared with heparin plus glycoprotein IIb-IIIa inhibitors, resulted in significantly lower 30-day rates of net adverse clinical events (9.2% vs. 12.2%; relative risk, 0.75; p=0.005), major bleeding (4.9% vs. 8.3%; relative risk, 0.59; p<0.001), death from cardiac causes (1.8% vs. 2.9%; relative risk, 0.62; p=0.03) and death from all causes (2.1% vs. 3.1%; relative risk, 0.66; p=0.047). The rates of re-infarction, target-vessel revascularization, and stroke were not significantly different between the two groups. The overall rate of stent thrombosis at 30 days did not differ significantly between the two groups (2.5% with bivalirudin and 1.9% in the other arm, p=0.30) but bivalirudin use was associated with increased risk of stent thrombosis within first 24 hours.
correspondence: Dr. Manish Bansal, Sr. Consultant Cardiologist, Indraprastha Apollo Hospital, New Delhi, INDIA
Email: manishaiims@hotmail.com
|
 |
Perspective:
In patients with STEMI undergoing primary PCI, unfractionated heparin with glycoprotein IIb-IIIa inhibitors is currently the preferred anticoagulant regimen. Bivalirudin, which is a newer anticoagulant has been evaluated in patients with chronic stable angina, unstable angina and non-ST elevation myocardial infarction undergoing PCI and has shown favorable results. This trial extended its use in the setting of STEMI. As expected, bivalirudin alone in this trial was associated with less risk of bleeding compared to a combination of heparin plus glycoprotein IIb-IIIa inhibitors. More importantly, at the same time, the risk of adverse ischemic events was not increased at 30 days. However, it must be remembered that almost 65% patients in the bivalirudin arm had received heparin before PCI which raises questions about the anti-thrombotic efficacy of bivalirudin alone. In addition, although 30-day rates of stent thrombosis were same in both the groups, there was significant increase in incidence of stent thrombosis within first 24 hours which also warrants consideration. Nonetheless, this trial has shown that bivalirudin alone may also be a reasonable option when selecting antithrombotic therapy in a patient with STEMI undergoing primary PCI.
JUPITER- Secondary analyses (Justification for the Use of Statins in Prevention: an Intervention Trial Evaluating Rosuvastatin)
Ridker PM, et al. Presented at the ACC Annual meeting 2009
JUPITER trial which was published recently had shown that 20 mg rosuvastatin given daily to apparently healthy individuals with normal LDL-C levels (<130 mg/dl) but elevated hs-CRP levels (> 2 mg/L) resulted in significant improvements in clinical outcomes. Two additional, clinically important findings from this study were presented in the recent ACC annual meeting and are discussed here.
Significance of dual treatment targets
JUPITER was the first study to prospectively examine the benefits of achieving dual targets with statin therapy. It showed that the patients achieving dual targets of lowering LDL-C to <70 mg/dl and hs-CRP to <2 mg/l derived significantly greater reduction in adverse CV outcomes as compared to those achieving only one of these two targets. In addition, the trial also showed that at least 50% reduction in both LDL-C and hs-CRP from the baseline values produced much greater benefit that proportionately less reduction in these markers, irrespective of the final levels achieved. Similar effects were observed after adjustment for all available baseline clinical characteristics, including baseline LDL-C and hs-CRP levels, and also in analyses based upon ApoB or the ApoB: ApoA ratio rather than upon LDL-C. Finally, much more marked reduction in hs-CRP levels to <1 mg/l in combination with LDL lowering to <70 g/dl was shown to result in an even greater (79%) relative risk reduction in the composite primary end-point.
These findings suggest that in patients receiving statin therapy for primary prevention of CV disease, reductions in both LDL-C and hs-CRP should be considered as the goals for a successful therapy.
Rosuvastatin and venous thromboembolism
Since many of the risk factors for arterial thrombosis are also associated with increased risk of VTE, it was postulated that statins could reduce incidence of VTE also in addition to their known beneficial effects on atherosclerotic vascular disease. However, previous observational studies have shown mixed results.
In JUPITER trial, over a median follow-up of 1.9 years, symptomatic VTE occurred in 94 participants: 34 in the rosuvastatin group and 60 in the placebo group (0.18 versus 0.32 events per 100 person-years respectively, hazard ratio with rosuvastatin 0.57; p=0.007). The corresponding rates for
|
unprovoked VTE were 0.10 and 0.17 (hazard ratio, 0.61; p=0.09) and for provoked VTE were 0.08 and 0.16 (hazard ratio, 0.52; p=0.03). There was no difference in the incidence of pulmonary embolism in the two groups but deep-vein thrombosis alone was significantly reduced in the rosuvastatin group (0.09 versus 0.20 events per 100 person-years respectively, hazard ratio 0.45; p=0.004). No significant differences were seen between treatment groups in the rates of bleeding episodes.
POISE [Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (POISE trial): a randomized controlled trial]
POISE study group. Lancet 2008;371:1839-47.
Trial summary:
The POISE trial was a randomized, double-blind, placebo-controlled trial, conducted to evaluate efficacy of peri-operative use of beta-blockers in reducing risk of CV events in patients undergoing major non-cardiac surgery.
Over 8000 patients with, or at risk of, atherosclerotic disease who were undergoing noncardiac surgery were randomized to receive extended-release metoprolol succinate (n = 4,174) or placebo (n = 4,177). Study drug was given 2 to 4 hours prior to surgery and for the next 30 days. Primary endpoint was major CV events (defined as CV death, MI, or cardiac arrest through 30 days).
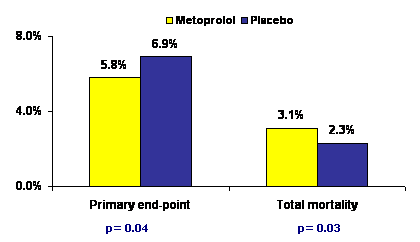
Metoprolol reduced the incidence of primary endpoint of CV death, MI, or cardiac arrest (5.8% vs. 6.9%, HR 0.83, p = 0.04). The benefit was achieved mainly by a marked reduction in nonfatal MI (3.6% vs. 5.1%, HR 0.70, p = 0.0007). However, the risk of hemodynamic complications (significant hypotension or bradycardia), stroke (1.0% vs. 0.5%, HR 2.17, p = 0.005) and overall mortality (3.1% vs. 2.3%, HR 1.33 p = 0.03) increased significantly in the metoprolol group.
In conclusion, in view of increased risk of death, stroke, and severe hypotension with metoprolol, routine prophylactic therapy does not appear to be a safe approach to reducing CV events in this population.
|
Perspective:
Prevention of adverse CV events is a major therapeutic concern in patients undergoing major noncardiac surgery. Although prophylactic use of beta-blockers is a standard practice in this setting, previous studies have shown mixed results. In the present trial, metoprolol use was associated with increase risk of death and stroke. However, a relatively higher dose of beta-blocker was used in this trial as compared to the previous studies and the beta-blocker therapy was initiated only 2-4 hours before surgery. Both these factors could have been responsible for these negative results. Therefore, the findings of this trial imply that acute administration of higher dose beta-blocker prior to non-cardiac surgery is detrimental and should be avoided. However, beta-blockers, when initiated well before surgery in patients at risk of having adverse CV events, may still be associated with overall benefit.
PROTECT-AF (Percutaneous Left Atrial Appendage Closure Versus Warfarin for Stroke Prevention in Atrial Fibrillation)
Holmes D, et al. Presented at the ACC Annual meeting 2009
Trial summary:
The PROTECT-AF trial was conducted to determine whether left atrial appendage closure with Watchman device followed by discontinuation of warfarin therapy in patients with AF was a safe and feasible option.
In this trial, 707 patients with non-valvular AF who were candidates for anticoagulation with warfarin but did not have any other indication for warfarin therapy were randomized to implantation of Watchman left-atrial appendage closure device followed by discontinuation of warfarin in 45 days (n = 463) versus continued warfarin therapy (n = 244). Clinical follow-up was planned for up to 5 years.

The incidence of primary efficacy outcome which was a composite of CV death, stroke, or systemic embolism was 3.4 events per 100 patient-years in the device group versus 5.0 events per 100 patient-years in the control group (p for noninferiority < 0.05). Incidence of all strokes was 3.4 per 100 patient-years versus 3.6 per 100 patient-years (p < 0.05 for noninferiority), respectively. There was one hemorrhagic stroke in the device group versus six in the warfarin group (p < 0.05 for superiority). The primary composite safety outcome was 8.7 events per 100 patient-years in the device group versus 4.2 events per 100 patient-years in the control group (p for superiority < 0.05). This was primarily related to complications (pericardial effusions) at the time of device implant.
Perspective:
Atrial fibrillation is the commonest form of cardiac arrhythmia and stroke is the number one cause of long-term disability and the third leading cause of death in patients with AF. Although anticoagulation with warfarin is the standard therapy in patients with AF, it is associated with increased risk of bleeding and requires frequent monitoring and dose-adjustments. The PROTECT-AF trial has demonstrated that use of Watchman device can afford effective protection against ischemic strokes with much lower risk of hemorrhagic strokes. However, procedural safety of this device is an area of concern which needs to be addressed in further trials before it could be considered an acceptable therapeutic option in these patients.
SAPPHIRE-3 year results (The Stenting and Angioplasty with Protection in Patients at High Risk for Endarterectomy study)
Gurm HS, et al. N Engl J Med 2008;358:1572-79
Trial summary:
This trial compared safety and efficacy of carotid stenting using embolic protection with carotid endarterectomy in management of occlusive carotid artery disease.
In this trial, 334 patients who had either a symptomatic carotid artery stenosis of at least 50% or an asymptomatic stenosis of at least 80% and were at high-surgical risk were randomized to undergo carotid artery stenting with the use of an emboli-protection device or carotid endarterectomy. The pre-specified major secondary end point at 3 years was a composite of death, stroke, or MI within 30 days after the procedure or death or ipsilateral stroke between 31 days and 1080 days (3 years).
At 3 years, the major secondary end point occurred in 41 patients in the stenting group (cumulative incidence 24.6%; Kaplan–Meier estimate 26.2%) and 45 patients in the endarterectomy group (cumulative incidence 26.9%; Kaplan–Meier estimate 30.3%, p =0.71). There was no difference in the mortality (20.0% stenting versus 24.2% surgery group; p = 0.68) or incidence of stroke at 3 years (10.1% stenting versus 10.7% surgery group; p = 0.77). Most of the mortality in both the groups resulted from non-cardiac causes. Target-vessel revascularization was infrequent in both the groups.
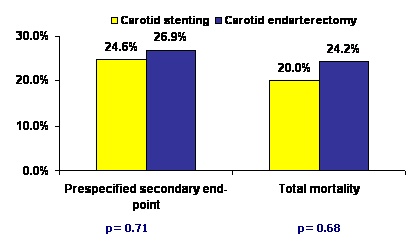
In conclusion, this trial showed that in patients with severe carotid artery stenosis and increased surgical risk, carotid stenting with embolic protection produces similar long-term results (including cumulative stroke risk and mortality) compared to surgery.
Perspective:
|
However, it must be noted that adequate technical expertise and use of appropriate devices are necessary to reproduce same results in the clinical practice.
SYNTAX (Percutaneous coronary intervention versus coronary-artery bypass grafting for severe coronary artery disease)
Serruys PW, et al. NEJM 2009;360:961-72
Trial summary:
This trial was conducted to evaluate role of PCI with DES in patients with triple-vessel disease and/or left-main CAD for which CABG still remains preferred revascularization strategy.
In this study, 1800 patients with three-vessel or left main CAD, for whom the local cardiac surgeon and interventional cardiologist determined that equivalent anatomical revascularization could be achieved with either CABG or PCI, were randomized to undergo one of the two treatment options. Patients were followed-up for 12-months after randomization. The primary end point was a composite of a major adverse cardiac or cerebrovascular event (i.e., death from any cause, stroke, MI, or repeat revascularization).
Patients in the PCI arm received 4.6 ± 2.3 paclitaxel-eluting stents on average whereas those in the CABG arm received 2.8 ± 0.7 grafts (an arterial graft in at least 97.3% patients). At the end of 12-month follow-up period, incidence of primary end-point was significantly higher in the PCI group (17.8% vs. 12.4% for CABG; p=0.002), mainly because of an increased rate of repeat revascularization in these patients (13.5% vs. 5.9%, p<0.001). Overall mortality and incidence of myocardial infarction were not different in the two groups. However, stroke was significantly more likely to occur with CABG (2.2%, vs. 0.6% with PCI; p=0.003).
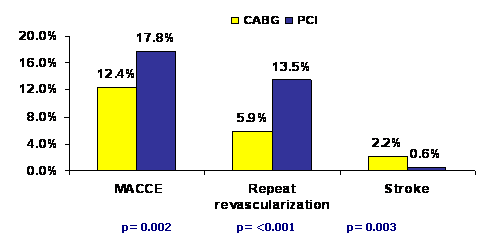
It was concluded that CABG, as compared with PCI, was associated with a lower rate of major adverse cardiac or cerebrovascular events at 1 year among patients with three-vessel or left main CAD (or both) and should therefore remain the standard of care for such patients.
Perspective:
SYNTAX trial was a large-scale randomized trial which, unlike previous trial on this issue, had enrolled ‘all-comers’. This coupled with the use of current state-of-the-art PCI and CABG techniques make this trial particularly relevant to current clinical practice. As mentioned, the trial showed that PCI with DES resulted in similar CV outcomes, except for the need for repeat revascularization, in patients with extensive CAD which was hitherto considered to be best treated with CABG. However, some practical issues need to be taken in to consideration. First, >4 paclitaxel-eluting stents were used on an average in each patient in this study which has major financial implications, esp. in India. Second, use of these many DES implies that dual anti-platelet therapy be continued for long-term, probably life-long which has its own problems. Third, long-term (>1 year) safety of PCI in these patients in not yet established. Thus in conclusion, CABG still remains preferred treatment strategy in patients with triple-vessel disease and/or left-main CAD. However, PCI with DES offers a solution in patients who are high surgical risk due to co-morbidities or who have less complex anatomical features and do not wish to undergo CABG.
|
TIPS (The Indian Polycap Study)
TIPS Investigators. Lancet 2009;373:1341-51
Trial summary:
The TIPS trial was conducted to determine efficacy and safety of a multi-drug formulation in controlling CV risk factors in patients without existing CV disease.
Over 2000 individuals without CV disease but with one major CV risk factor were randomized to receive a Polycap (containing hydrochlorothiazide 12.5 mg, atenolol 50 mg, ramipril 5 mg, simvastatin 20 mg, and aspirin 80 mg) or its individual components in different combinations for a period of 12 weeks.
At the end of the follow-up, blood pressure lowering with the Polycap was similar to the groups receiving three blood pressure drugs (7.4/5.6 mm Hg vs. 6.6/4.8 mm Hg, p < 0.0001 for noninferiority). Similarly, heart rate lowering was similar in the Polycap and other atenolol groups (7.0 bpm, 95% confidence interval 6-8). However, LDL-C lowering was greater with simvastatin alone compared with the Polycap (-0.83 vs. -0.70 mmol/L, p=0.04) and although the Polycap significantly reduced urinary 11-dehydrothromboxane B2 levels, it did not achieve prespecified noninferiority criteria compared to aspirin alone (p = 0.57). The Polycap was well tolerated in this study.
In conclusion, this study showed that the Polycap was safe and was non-inferior to its individual components in terms of blood pressure and heart rate lowering but was slightly less effective in LDL-C reduction and platelet inhibition than simvastatin and aspirin alone.
Recently, a concept of multi-drug treatment of all individuals in the form of a polypill, irrespective of their CV risk factor profile, has been proposed with a view to provide an effective means for prevention of CV disease. However, there are several questions that remain unanswered including the composition of such a polypill, its efficacy, potential of drug-drug interactions, effect on long-term outcomes, cost-effectiveness, etc. The present study was conducted as the first step towards answering some of these questions pertaining to the efficacy and safety of a polypill. In this study, the Polycap was found to have similar blood pressure and heart rate lowering effects as its individual components but was inferior to simvastatin and aspirin in LDL-C reduction and platelet inhibition respectively. No safety issues were observed with the Polycap in this study. However, since this was only a short-term study, long-term safety of such a formulation remains to be assessed. In addition, as the primary aim of the polypill strategy is to reduce CV events in a cost-effective manner, large scale clinical trials are needed to address this issue.
ABBREVIATIONS
AF: atrial fibrillation
CABG: coronary artery bypass grafting
CAD: coronary artery disease
CV: cardiovascular
DES: drug-eluting stents
hs-CRP: high-sensitivity C-reactive protein
LDL-C: low-density lipoprotein cholesterol
MI: myocardial infarction
PCI: percutaneous coronary intervention
STEMI: ST-elevation myocardial infarction
VTE: venous thromboembolism
|